Page 278 - Elements of Chemical Reaction Engineering 3rd Edition
P. 278
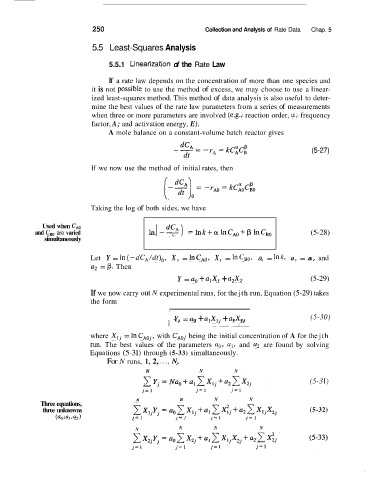
250 Collection and Analysis of Rate Data Chap. 5
5.5 Least-Squares Analysis
5.5.1 Linear’zation of the Rate Law
If a rate law depends on the concentration of more than one species and
it is not possibIe to use the method of excess, we may choose to use a linear-
ized least-squares method. This method of data analysis is also useful to deter-
mine the best values of the rate law parameters from a series of measurements
when three or more parameters are involved (e.& reaction order, a; frequency
factor, A; and activation energy, E).
A mole balance on a constant-volume batch reactor gives
(5-27)
If we now use the method of initial rates, then
Taking the log of both sides, we have
Used when C,,
and C,, are varied (5-28)
simultaneously
Let Y = ln(-dC,/dt),, X, = hCA0, X, = InC,,, a, = Ink, a, = a, and
a, = p. Then
Y = a, + alX, + a,X, (5-29)
If we now carry out N experimental runs, for the jth run, Equation (5-29) takes
the form
Y, = a, + a,X,, + a,X2, (5-30)
where Xlj = In CAO,, with CAOj being the initial concentration of A for the jth
run. The best values of the parameters a,, a,, and a, are found by solving
Equations (5-3 1) through (5-33) simultaneously.
For N runs, 1, 2, ..., N,
N N N
(5-3 1)
N N N N
Three equations,
three unknowns (5-32)
(ao, a,, a2 ) j= 1 j= 1 j= 1 j= 1
N N N N