Page 189 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 189
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-69 July 19, 2001 22:50
Microanalytical Assays 689
and therefore do not affect the potential of the electrode The extent of reaction, and therefore the glutamine
inside the glass electrode. Thus the glass pH electrode concentration, was determined by measuring the ammo-
is very robust device since it can be utilized in diverse nium content in the sensor. This was achieved with an
environments. ammonia electrode. Also shown is the response of the
One of the limitations of using electrodes as detectors sensor in the presence of various potential interfering
is that many electroactive substances do not react at the amino acids, which made a very minor contribution to
electrode surface easily, causing variations in response the sensor response. This example illustrates the superb
with time. This problem can be overcome by utilizing selectivity potential of enzymes due to their high degree of
highly reversibly electroactive substances (known as me- specificity. The response of the sensor is over three orders
diators) as intermediates between the analyte and elec- of magnitude of glutamine concentration, showing the
trode. Some common mediators are ferrocene, phenazine wide dynamic range of potentiometric based transducers.
methosulfate, and benzylviologen. Since different organic
materials have different potentials where they react in
the electrode, is necessary to choose a mediator that has VI. OPTICALLY BASED BIOSENSORS
an oxidation/reduction potential that is near the oxida-
tion/reduction potential of the analyte. The use of optical methods became popularized after the
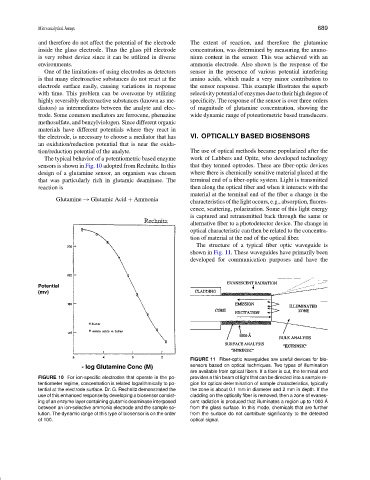
The typical behavior of a potentiometric based enzyme work of Lubbers and Opitz, who developed technology
sensors is shown in Fig. 10 adopted from Rechnitz. In this that they termed optrodes. These are fiber-optic devices
design of a glutamine sensor, an organism was chosen where there is chemically sensitive material placed at the
that was particularly rich in glutamic deaminase. The terminal end of a fiber-optic system. Light is transmitted
reaction is then along the optical fiber and when it interacts with the
material at the terminal end of the fiber a change in the
Glutamine −→ Glutamic Acid + Ammonia
characteristics of the light occurs, e.g., absorption, fluores-
cence, scattering, polarization. Some of this light energy
is captured and retransmitted back through the same or
alternative fiber to a photodetector device. The change in
optical characteristic can then be related to the concentra-
tion of material at the end of the optical fiber.
The structure of a typical fiber optic waveguide is
shown in Fig. 11. These waveguides have primarily been
developed for communication purposes and have the
FIGURE 11 Fiber-optic waveguides are useful devices for bio-
sensors based on optical techniques. Two types of illumination
are available from optical fibers. If a fiber is cut, the terminal end
FIGURE 10 For ion-specific electrodes that operate in the po- provides a thin beam of light that can be directed into a sample re-
tentiometer regime, concentration is related logarithmically to po- gion for optical determination of sample characteristics, typically
tential at the electrode surface. Dr. G. Rechnitz demonstrated the the zone is about 0.1 mm in diameter and 2 mm in depth. If the
use of this enhanced response by developing a biosensor consist- cladding on the optically fiber is removed, then a zone of evanes-
ing of an enzyme layer containing glutamic deaminase interposed cent radiation is produced that illuminates a region up to 1000 ˚ A
between an ion-selective ammonia electrode and the sample so- from the glass surface. In this mode, chemicals that are further
lution. The dynamic range of this type of biosensor is on the order from the surface do not contribute significantly to the detected
of 100. optical signal.