Page 190 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 190
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-69 July 19, 2001 22:50
690 Microanalytical Assays
outstanding characteristic that the amount of light loss due
to absorption and scattering is minuscule for path lengths
on the order of meters. Also, the diameter of the fibers can
be as small as 10 µ, although 150 µ is more usual. Thus,
these optical waveguides can be used for transmitting opti-
cal signals almost without lost over the distances that one
might need for biosensor monitoring devices. Hundreds
of types of optical fibers are available in many different
sizes and of materials, so that there is a large selection
from which to choose.
There are essentially two modes by which light energy
is dissipated from these waveguides, as shown in Fig. 11.
First, light emanates from distal or cut end of the fiber,
much in the same manner as the beam of light coming
out of a flashlight. The intensity of this light is a func-
tion of distance from the end face and radial position off
the center axis of the fiber. The magnitude of the inten-
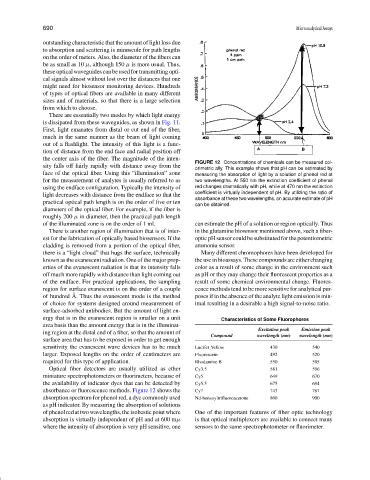
FIGURE 12 Concentrations of chemicals can be measured col-
sity falls off fairly rapidly with distance away from the
orimetric ally. This example shows that pH can be estimated by
face of the optical fiber. Using this “illumination” zone measuring the absorption of light by a solution of phenol red at
for the measurement of analytes is usually referred to as two wavelengths. At 550 nm the extinction coefficient of phenol
using the endface configuration. Typically the intensity of red changes dramatically with pH, while at 470 nm the extinction
coefficient is virtually independent of pH. By utilizing the ratio of
light decreases with distance from the endface so that the
absorbance at these two wavelengths, an accurate estimate of pH
practical optical path length is on the order of five or ten
can be obtained.
diameters of the optical fiber. For example, if the fiber is
roughly 200 µ in diameter, then the practical path length
of the illuminated zone is on the order of 1 ml. can estimate the pH of a solution or region optically. Thus
There is another region of illumination that is of inter- in the glutamine biosensor mentioned above, such a fiber-
est for the fabrication of optically based biosensors. If the optic pH sensor could be substituted for the potentiometric
cladding is removed from a portion of the optical fiber, ammonia sensor.
there is a “light cloud” that hugs the surface, technically Many different chromophores have been developed for
known as the evanescent radiation. One of the major prop- the use in bioassays. These compounds are either changing
erties of the evanescent radiation is that its intensity falls color as a result of some change in the environment such
off much more rapidly with distance than light coming out as pH or they may change their fluorescent properties as a
of the endface. For practical applications, the sampling result of some chemical environmental change. Fluores-
region for surface evanescent is on the order of a couple cence methods tend to be more sensitive for analytical pur-
˚
of hundred A. Thus the evanescent mode is the method poses if in the absence of the analyte light emission is min-
of choice for systems designed around measurement of imal resulting in a desirable a high signal-to-noise ratio.
surface-adsorbed antibodies. But the amount of light en-
ergy that is in the evanescent region is smaller on a unit Characteristics of Some Fluorophores
area basis than the amount energy that is in the illuminat-
Excitation peak Emission peak
ing region at the distal end of a fiber, so that the amount of
Compound wavelength (nm) wavelength (nm)
surface area that has to be exposed in order to get enough
sensitivity the evanescent wave devices has to be much Lucifer Yellow 430 540
larger. Exposed lengths on the order of centimeters are Fluorescein 492 520
required for this type of application. Rhodamine B 550 585
Optical fiber detectors are usually utilized as ether Cy3.5 581 596
miniature spectrophotometers or fluorimeters, because of Cy5 649 670
the availability of indicator dyes that can be detected by Cy5.5 675 694
absorbance or fluorescence methods. Figure 12 shows the Cy7 743 767
absorption spectrum for phenol red, a dye commonly used Nd-benzoyltrifluoroacetone 800 900
as pH indicator. By measuring the absorption of solutions
ofphenolredattwowavelengths,theisobesticpointwhere One of the important features of fiber optic technology
absorption is virtually independent of pH and at 600 mµ is that optical multiplexers are available to connect many
where the intensity of absorption is very pH sensitive, one sensors to the same spectrophotometer or fluorimeter.