Page 81 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 81
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN002G-67 May 25, 2001 20:8
Bioreactors 263
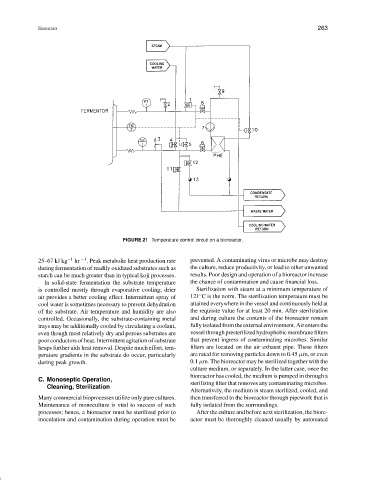
FIGURE 21 Temperature control circuit on a bioreactor.
25–67 kJ kg −1 hr −1 . Peak metabolic heat production rate prevented. A contaminating virus or microbe may destroy
during fermentation of readily oxidized substrates such as the culture, reduce productivity, or lead to other unwanted
starch can be much greater than in typical koji processes. results. Poor design and operation of a bioreactor increase
In solid-state fermentation the substrate temperature the chance of contamination and cause financial loss.
is controlled mostly through evaporative cooling; drier Sterilization with steam at a minimum temperature of
◦
air provides a better cooling effect. Intermittent spray of 121 C is the norm. The sterilization temperature must be
cool water is sometimes necessary to prevent dehydration attained everywhere in the vessel and continuously held at
of the substrate. Air temperature and humidity are also the requisite value for at least 20 min. After sterilization
controlled. Occasionally, the substrate-containing metal and during culture the contents of the bioreactor remain
trays may be additionally cooled by circulating a coolant, fully isolated from the external environment. Air enters the
even though most relatively dry and porous substrates are vessel through presterilized hydrophobic membrane filters
poor conductors of heat. Intermittent agitation of substrate that prevent ingress of contaminating microbes. Similar
heaps further aids heat removal. Despite much effort, tem- filters are located on the air exhaust pipe. These filters
perature gradients in the substrate do occur, particularly are rated for removing particles down to 0.45 µm, or even
during peak growth. 0.1 µm. The bioreactor may be sterilized together with the
culture medium, or separately. In the latter case, once the
bioreactor has cooled, the medium is pumped in through a
C. Monoseptic Operation,
sterilizing filter that removes any contaminating microbes.
Cleaning, Sterilization
Alternatively, the medium is steam sterilized, cooled, and
Many commercial bioprocesses utilize only pure cultures. then transferred to the bioreactor through pipework that is
Maintenance of monoculture is vital to success of such fully isolated from the surroundings.
processes; hence, a bioreactor must be sterilized prior to After the culture and before next sterilization, the biore-
inoculation and contamination during operation must be actor must be thoroughly cleaned usually by automated