Page 416 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 416
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-427 July 6, 2001 20:25
Metalorganic Chemical Vapor Deposition 501
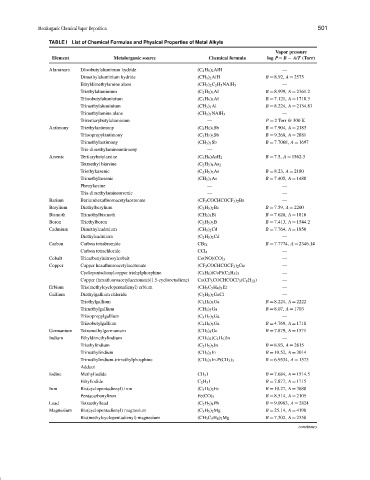
TABLE I List of Chemical Formulas and Physical Properties of Metal Alkyls
Vapor pressure
Element Metalorganic source Chemical formula log P= B − A/T (Torr)
=
Aluminum Diisobutylaluminum hydride (C 4 H 9 ) 2 AlH —
Dimethylaluminium hydride (CH 3 ) 2 AlH B = 8.92, A = 2575
Ethyldimethylamine alane (CH 3 ) 2 C 2 H 5 NAlH 3 —
Triethylaluminium (C 2 H 5 ) 3 Al B = 8.999, A = 2361.2
Triisobutylaluminium (C 4 H 9 ) 3 Al B = 7.121, A = 1710.3
Trimethylaluminium (CH 3 ) 3 Al B = 8.224, A = 2134.83
Trimethylamine alane (CH 3 ) 3 NAlH 3 —
Tritertiarybutylaluminum — P = 2 Torr @ 300 K
Antimony Triethylantimony (C 2 H 5 ) 3 Sb B = 7.904, A = 2183
Triisopropylantimony (C 3 H 7 ) 3 Sb B = 9.268, A = 2881
Trimethylantimony (CH 3 ) 3 Sb B = 7.7068, A = 1697
Tris-dimethylaminoantimony —
Arsenic Tertiarybutylarsine (C 4 H 9 )AsH 2 B = 7.5, A = 1562.3
Tetraethyl biarsine (C 2 H 5 ) 4 As 2
Triethylarsenic (C 2 H 5 ) 3 As B = 8.23, A = 2180
Trimethylarsenic (CH 3 ) 3 As B = 7.405, A = 1480
Phenylarsine — —
Tris-dimethylaminoarsenic — —
Barium Bariumhexafluoroacetylacetonate (CF 3 COCHCOCF 3 ) 2 Ba —
Berylium Diethylberylium (C 2 H 5 ) 2 Be B = 7.59, A = 2200
Bismuth Trimethylbismuth (CH 3 ) 3 Bi B = 7.628, A = 1816
Boron Triethylboron (C 2 H 5 ) 3 B B = 7.413, A = 1544.2
Cadmium Dimethylcadmium (CH 3 ) 2 Cd B = 7.764, A = 1850
Diethylcadmium (C 2 H 5 ) 2 Cd —
Carbon Carbon tetrabromide CBr 4 B = 7.7774, A = 2346.14
Carbon tetrachloride CCl 4 —
Cobalt Tricarbonylnitrosylcobalt Co(NO)(CO) 3 —
Copper Copper hexafluoroacetylacetonate (CF 3 COCHCOCF 3 ) 2 Cu —
Cyclopentadienylcopper triehylphosphine (C 5 H 5 )(CuP)(C 2 H 5 ) 3 —
Copper (hexafluoroacetylacetonate)(1.5-cyclooctadiene) Cu(CF 3 COCHCOCF 3 (C 8 H 12 ) —
Erbium Tris(methylcyclopentadienyl) erbium (CH 3 C 5 H 4 ) 3 Er —
Gallium Diethylgallium chloride (C 2 H 5 ) 2 GaCl —
Triethylgallium (C 2 H 5 ) 3 Ga B = 8.224, A = 2222
Trimethylgallium (CH 3 ) 3 Ga B = 8.07, A = 1703
Triisopropylgallium (C 3 H 7 ) 3 Ga —
Triisobutylgallium (C 4 H 9 ) 3 Ga B = 4.769, A = 1718
Germanium Tetramethylgermanium (CH 3 ) 4 Ge B = 7.879, A = 1571
Indium Ethyldimethylindium (CH 3 ) 2 (C 2 H 5 )In —
Triethylindium (C 2 H 5 ) 3 In B = 8.93, A = 2815
Trimethylindium (CH 3 ) 3 In B = 10.52, A = 3014
Trimethylindium-trimethylphosphine (CH 3 ) 3 In-P(CH 3 ) 3 B = 6.9534, A = 1573
Adduct
Iodine Methyliodide CH 3 I B = 7.684, A = 1514.5
Ethyliodide C 2 H 5 I B = 7.877, A = 1715
Iron Bis(cyclopentadienyl) iron (C 5 H 5 ) 2 Fe B = 10.27, A = 3680
Pentacarbonyliron Fe(CO) 5 B = 8.514, A = 2105
Lead Tetraethyllead (C 2 H 5 ) 4 Pb B = 9.0983, A = 2824
Magnesium Bis(cyclopentadienyl) magnesium (C 5 H 5 ) 2 Mg B = 25.14, A = 4198
Bis(methylcyclopentadienyl) magnesium (CH 3 C 5 H 4 ) 2 Mg B = 7.302, A = 2358
continues