Page 417 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 417
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-427 July 6, 2001 20:25
502 Metalorganic Chemical Vapor Deposition
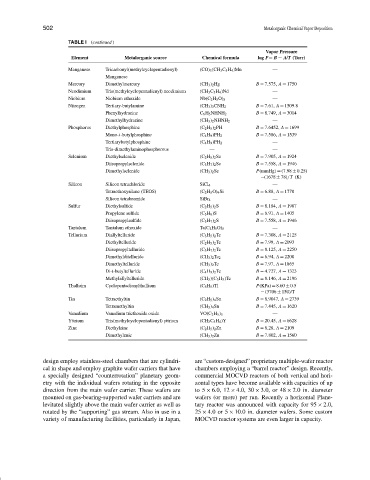
TABLE I (continued )
Vapor Pressure
Element Metalorganic source Chemical formula log P= B − A/T (Torr)
=
Manganese Tricarbonyl(methylcyclopentadienyl) (CO) 3 (CH 3 C 5 H 4 )Mn —
Manganese
Mercury Dimethylmercury (CH 3 ) 2 Hg B = 7.575, A = 1750
Neodimium Tris(methylcyclopentadienyl) neodimium (CH 3 C 5 H 4 )Nd —
Niobium Niobium ethoxide Nb(C 2 H 5 O) 5 —
Nitrogen Tertiary-butylamine (CH 3 ) 3 CNH 2 B = 7.61, A = 1509.8
Phenylhydrazine C 6 H 5 NHNH 2 B = 8.749, A = 3014
Dimethylhydrazine (CH 3 ) 2 NHNH 2 —
Phosphorus Diethylphosphine (C 2 H 5 ) 2 PH B = 7.6452, A = 1699
Mono-t-butylphosphine (C 4 H 9 )PH 2 B = 7.586, A = 1539
Tertiarybutylphosphine (C 4 H 9 )PH 2 —
Tris-dimethylaminophosphorous — —
Selenium Diethylselenide (C 2 H 5 ) 2 Se B = 7.905, A = 1924
Diisopropylselenide (C 3 H 7 ) 2 Se B = 7.558, A = 1946
Dimethylselenide (CH 3 ) 2 Se P(mmHg) = (7.98 ± 0.25)
−(1678 ± 78)/T (K)
Silicon Silicon tetrachloride SiCl 4 —
Tetraethoxysilane (TEOS) (C 2 H 5 O) 4 Si B = 6.88, A = 1770
Silicon tetrabromide SiBr 4 —
Sulfur Diethylsulfide (C 2 H 5 ) 2 S B = 8.184, A = 1907
Propylene sulfide (C 3 H 6 )S B = 6.91, A = 1405
Diisopropylsulfide (C 3 H 7 ) 2 S B = 7.558, A = 1946
Tantalum Tantalum ethoxide Ta(C 2 H 5 O) 5 —
Tellurium Diallyltelluride (C 3 H 5 ) 2 Te B = 7.308, A = 2125
Diethyltelluride (C 2 H 5 ) 2 Te B = 7.99, A = 2093
Diisopropyltelluride (C 3 H 7 ) 2 Te B = 8.125, A = 2250
Dimethylditelluride (CH 3 ) 2 Te 2 B = 6.94, A = 2200
Dimethyltelluride (CH 3 ) 2 Te B = 7.97, A = 1865
Di-t-butyltelluride (C 4 H 9 ) 2 Te B = 4.727, A = 1323
Methylallyltelluride (CH 3 )(C 3 H 5 )Te B = 8.146, A = 2196
Thalluim Cyclopentadienylthallium (C 5 H 5 )Tl P(KPa) = 8.60 ± 0.5
− (3706 ± 150)/T
Tin Tetraethyltin (C 2 H 5 ) 4 Sn B = 8.9047, A = 2739
Tetramethyltin (CH 3 ) 4 Sn B = 7.445, A = 1620
Vanadium Vanadium triethoxide oxide VO(C 2 H 5 ) 3 —
Yttrium Tris(methylcyclopentadienyl) yttrium (CH 3 C 5 H 4 )Y B = 20.45, A = 6628
Zinc Diethylzinc (C 2 H 5 ) 2 Zn B = 8.28, A = 2109
Dimethylznic (CH 3 ) 2 Zn B = 7.802, A = 1560
design employ stainless-steel chambers that are cylindri- are “custom-designed” proprietary multiple-wafer reactor
cal in shape and employ graphite wafer carriers that have chambers employing a “barrel reactor” design. Recently,
a specially designed “counterrotation” planetary geom- commercial MOCVD reactors of both vertical and hori-
etry with the individual wafers rotating in the opposite zontal types have become available with capacities of up
direction from the main wafer carrier. These wafers are to 5 × 6.0, 12 × 4.0, 30 × 3.0, or 48 × 2.0 in. diameter
mounted on gas-bearing-supported wafer carriers and are wafers (or more) per run. Recently a horizontal Plane-
levitated slightly above the main wafer carrier as well as tary reactor was announced with capacity for 95 × 2.0,
rotated by the “supporting” gas stream. Also in use in a 25 × 4.0or5 × 10.0 in. diameter wafers. Some custom
variety of manufacturing facilities, particularly in Japan, MOCVD reactor systems are even larger in capacity.