Page 414 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 414
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-427 July 6, 2001 20:25
Metalorganic Chemical Vapor Deposition 499
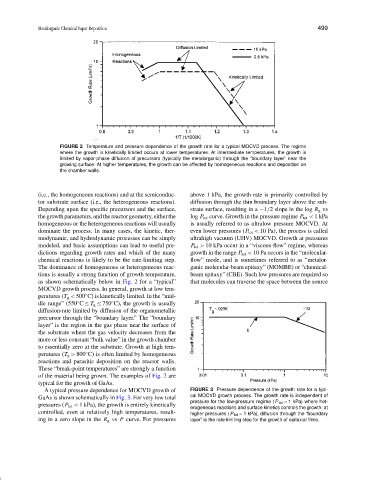
FIGURE 2 Temperature and pressure dependence of the growth rate for a typical MOCVD process. The regime
where the growth is kinetically limited occurs at lower temperatures. At intermediate temperatures, the growth is
limited by vapor-phase diffusion of precursors (typically the metalorganic) through the “boundary layer” near the
growing surface. At higher temperatures, the growth can be affected by homogeneous reactions and deposition on
the chamber walls.
(i.e., the homogeneous reactions) and at the semiconduc- above 1 kPa, the growth rate is primarily controlled by
tor substrate surface (i.e., the heterogeneous reactions). diffusion through the thin boundary layer above the sub-
Depending upon the specific precursors and the surface, strate surface, resulting in a −1/2 slope in the log R g vs
the growth parameters, and the reactor geometry, either the log P tot curve. Growth in the pressure regime P tot < 1kPa
homogeneous or the heterogeneous reactions will usually is usually referred to as ultralow pressure MOCVD. At
dominate the process. In many cases, the kinetic, ther- even lower pressures (P tot < 10 Pa), the process is called
modynamic, and hydrodynamic processes can be simply ultrahigh vacuum (UHV) MOCVD. Growth at pressures
modeled, and basic assumptions can lead to useful pre- P tot > 10 kPa occur in a “viscous-flow” regime, whereas
dictions regarding growth rates and which of the many growth in the range P tot < 10 Pa occurs in the “molecular-
chemical reactions is likely to be the rate-limiting step. flow” mode, and is sometimes referred to as “metalor-
The dominance of homogeneous or heterogeneous reac- ganic molecular-beam epitaxy” (MOMBE) or “chemical-
tions is usually a strong function of growth temperature, beam epitaxy” (CBE). Such low pressures are required so
as shown schematically below in Fig. 2 for a “typical” that molecules can traverse the space between the source
MOCVD growth process. In general, growth at low tem-
◦
peratures (T g < 500 C) is kinetically limited. In the “mid-
dle range” (550 C ≤ T g ≤ 750 C), the growth is usually
◦
◦
diffusion-rate limited by diffusion of the organometallic
precursor through the “boundary layer.” The “boundary
layer” is the region in the gas phase near the surface of
the substrate where the gas velocity decreases from the
more or less constant “bulk value” in the growth chamber
to essentially zero at the substrate. Growth at high tem-
peratures (T g > 800 C) is often limited by homogeneous
◦
reactions and parasitic deposition on the reactor walls.
These “break-point temperatures” are strongly a function
of the material being grown. The examples of Fig. 2 are
typical for the growth of GaAs.
A typical pressure dependence for MOCVD growth of FIGURE 3 Pressure dependence of the growth rate for a typi-
GaAs is shown schematically in Fig. 3. For very low total cal MOCVD growth process. The growth rate is independent of
pressure for the low-pressure regime (P tot < 1 kPa) where het-
pressures (P tot < 1 kPa), the growth is entirely kinetically
erogeneous reactions and surface kinetics controls the growth; at
controlled, even at relatively high temperatures, result- higher pressures (P tot > 1 kPa), diffusion through the “boundary
ing in a zero slope in the R g vs P curve. For pressures layer” is the rate-limiting step for the growth of epitaxial films.