Page 134 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 134
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
482 Catalysis, Homogeneous
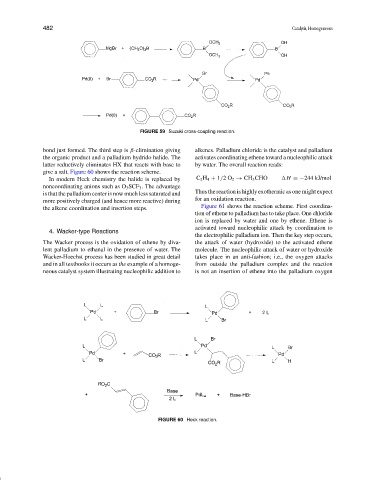
FIGURE 59 Suzuki cross-coupling reaction.
bond just formed. The third step is β-elimination giving alkenes. Palladium chloride is the catalyst and palladium
the organic product and a palladium hydrido halide. The activates coordinating ethene toward a nucleophilic attack
latter reductively eliminates HX that reacts with base to by water. The overall reaction reads:
give a salt. Figure 60 shows the reaction scheme.
In modern Heck chemistry the halide is replaced by C 2 H 4 + 1/2O 2 → CH 3 CHO H =−244 kJ/mol
noncoordinating anions such as O 3 SCF 3 . The advantage
Thus the reaction is highly exothermic as one might expect
is that the palladium center is now much less saturated and
for an oxidation reaction.
more positively charged (and hence more reactive) during
Figure 61 shows the reaction scheme. First coordina-
the alkene coordination and insertion steps.
tion of ethene to palladium has to take place. One chloride
ion is replaced by water and one by ethene. Ethene is
activated toward nucleophilic attack by coordination to
4. Wacker-type Reactions
the electrophilic palladium ion. Then the key step occurs,
The Wacker process is the oxidation of ethene by diva- the attack of water (hydroxide) to the activated ethene
lent palladium to ethanal in the presence of water. The molecule. The nucleophilic attack of water or hydroxide
Wacker-Hoechst process has been studied in great detail takes place in an anti-fashion; i.e., the oxygen attacks
and in all textbooks it occurs as the example of a homoge- from outside the palladium complex and the reaction
neous catalyst system illustrating nucleophilic addition to is not an insertion of ethene into the palladium oxygen
FIGURE 60 Heck reaction.