Page 141 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 141
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
Catalysis, Homogeneous 489
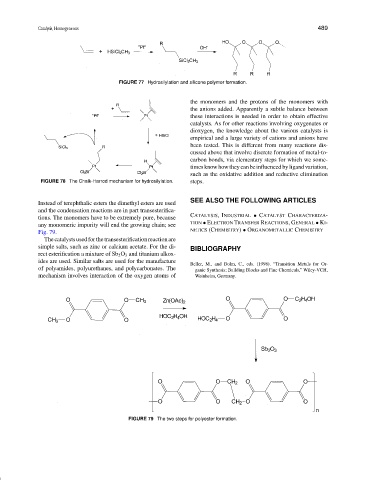
FIGURE 77 Hydrosilylation and silicone polymer formation.
the monomers and the protons of the monomers with
the anions added. Apparently a subtle balance between
these interactions is needed in order to obtain effective
catalysts. As for other reactions involving oxygenates or
dioxygen, the knowledge about the various catalysts is
empirical and a large variety of cations and anions have
been tested. This is different from many reactions dis-
cussed above that involve discrete formation of metal-to-
carbon bonds, via elementary steps for which we some-
timesknowhowtheycanbeinfluencedbyligandvariation,
such as the oxidative addition and reductive elimination
FIGURE 78 The Chalk-Harrod mechanism for hydrosilylation. steps.
SEE ALSO THE FOLLOWING ARTICLES
Instead of terephthalic esters the dimethyl esters are used
and the condensation reactions are in part transesterifica-
CATALYSIS,INDUSTRIAL • CATALYST CHARACTERIZA-
tions. The monomers have to be extremely pure, because
TION • ELECTRON TRANSFER REACTIONS,GENERAL • KI-
any monomeric impurity will end the growing chain; see
Fig. 79. NETICS (CHEMISTRY) • ORGANOMETALLIC CHEMISTRY
Thecatalystsusedforthetransesterificationreactionare
simple salts, such as zinc or calcium acetate. For the di- BIBLIOGRAPHY
rect esterification a mixture of Sb 2 O 3 and titanium alkox-
ides are used. Similar salts are used for the manufacture
Beller, M., and Bolm, C., eds. (1998). “Transition Metals for Or-
of polyamides, polyurethanes, and polycarbonates. The ganic Synthesis; Building Blocks and Fine Chemicals,” Wiley-VCH,
mechanism involves interaction of the oxygen atoms of Weinheim, Germany.
FIGURE 79 The two steps for polyester formation.