Page 46 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 46
P1: FYK/LSX Revised Pages P2: FWQ/FPW QC: FYD
Encyclopedia of Physical Science and Technology en001d42 April 28, 2001 15:9
770 Atomic Spectrometry
developing time, and chemicals for the development pro- III. ATOMIC EMISSION SPECTROMETRY
cess). Today, photomultipliers (PMT), photodiodes, and
array detectors completely dominate the photon detection A. Theoretical Background
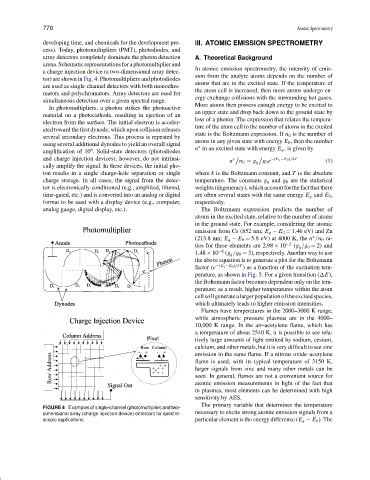
arena.Schematicrepresentationsforaphotomultiplierand
In atomic emission spectrometry, the intensity of emis-
a charge injection device (a two-dimensional array detec-
sion from the analyte atoms depends on the number of
tor) are shown in Fig. 4. Photomultipliers and photodiodes
atoms that are in the excited state. If the temperature of
are used as single-channel detectors with both monochro-
the atom cell is increased, then more atoms undergo en-
mators and polychromators. Array detectors are used for
ergy exchange collisions with the surrounding hot gases.
simultaneous detection over a given spectral range.
More atoms then possess enough energy to be excited to
In photomultipliers, a photon strikes the photoactive
an upper state and drop back down to the ground state by
material on a photocathode, resulting in ejection of an
loss of a photon. The expression that relates the tempera-
electron from the surface. The initial electron is acceler-
ture of the atom cell to the number of atoms in the excited
ated toward the first dynode, which upon collision releases
state is the Boltzmann expression. If n 0 is the number of
several secondary electrons. This process is repeated by
atoms in any given state with energy E 0 , then the number
using several additional dynodes to yield an overall signal ∗
6
amplification of 10 . Solid-state detectors (photodiodes n in an excited state with energy E q ,isgivenby
and charge injection devices), however, do not intrinsi- n ∗ n 0 = g q g 0 e −(E q −E 0 )/kT (1)
cally amplify the signal. In these devices, the initial pho-
ton results in a single charge-hole separation or single where k is the Boltzmann constant, and T is the absolute
charge storage. In all cases, the signal from the detec- temperature. The constants g q and g 0 are the statistical
tor is electronically conditioned (e.g., amplified, filtered, weights (degeneracy), which account for the fact that there
time-gated, etc.) and is converted into an analog or digital are often several states with the same energy E q and E 0 ,
format to be used with a display device (e.g., computer, respectively.
analog gauge, digital display, etc.). The Boltzmann expression predicts the number of
atoms in the excited state, relative to the number of atoms
in the ground state. For example, considering the atomic
emission from Cs (852 nm; E q − E 0 = 1.46 eV) and Zn
(213.8 nm; E q − E 0 = 5.8 eV) at 4000 K, the n /n 0 ra-
∗
tios for these elements are 2.98 × 10 −2 (g q /g 0 = 2) and
1.48 × 10 −6 (g q /g 0 = 3), respectively. Another way to use
the above equation is to generate a plot for the Boltzmann
factor (e −(E q −E 0 )/kT ) as a function of the excitation tem-
perature, as shown in Fig. 5. For a given transition ( E),
the Boltzmann factor becomes dependent only on the tem-
perature; as a result, higher temperatures within the atom
cellwillgeneratealargerpopulationoftheexcitedspecies,
which ultimately leads to higher emission intensities.
Flames have temperatures in the 2000–3000 K range,
while atmospheric pressure plasmas are in the 4000–
10,000 K range. In the air–acetylene flame, which has
a temperature of about 2540 K, it is possible to see rela-
tively large amounts of light emitted by sodium, cesium,
calcium, and other metals, but it is very difficult to see zinc
emission in the same flame. If a nitrous oxide–acetylene
flame is used, with its typical temperature of 3150 K,
larger signals from zinc and many other metals can be
seen. In general, flames are not a convenient source for
atomic emission measurements in light of the fact that
in plasmas, most elements can be determined with high
sensitivity by AES.
The primary variable that determines the temperature
FIGURE 4 Examples of single-channel (photomultiplier) and two-
dimensional array (charge injection device) detectors for spectro- necessary to excite strong atomic emission signals from a
scopic applications. particular element is the energy difference (E q − E 0 ). The