Page 53 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 53
P1: FYK/LSX Revised Pages P2: FWQ/FPW QC: FYD
Encyclopedia of Physical Science and Technology en001d42 April 28, 2001 15:9
Atomic Spectrometry 777
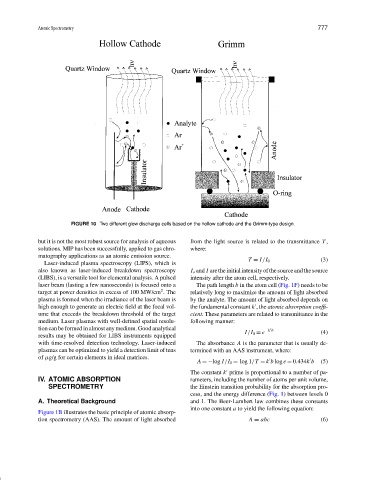
FIGURE 10 Two different glow discharge cells based on the hollow cathode and the Grimm-type design.
but it is not the most robust source for analysis of aqueous from the light source is related to the transmittance T ,
solutions. MIP has been successfully, applied to gas chro- where:
matography applications as an atomic emission source.
T = I/I 0 (3)
Laser-induced plasma spectroscopy (LIPS), which is
also known as laser-induced breakdown spectroscopy I o and I are the initial intensity of the source and the source
(LIBS), is a versatile tool for elemental analysis. A pulsed intensity after the atom cell, respectively.
laser beam (lasting a few nanoseconds) is focused onto a The path length b in the atom cell (Fig. 1F) needs to be
2
target at power densities in excess of 100 MW/cm . The relatively long to maximize the amount of light absorbed
plasma is formed when the irradiance of the laser beam is by the analyte. The amount of light absorbed depends on
high enough to generate an electric field at the focal vol- the fundamental constant k , the atomic absorption coeffi-
ume that exceeds the breakdown threshold of the target cient. These parameters are related to transmittance in the
medium. Laser plasmas with well-defined spatial resolu- following manner:
tion can be formed in almost any medium. Good analytical −k b
I/I 0 = e (4)
results may be obtained for LIBS instruments equipped
with time-resolved detection technology. Laser-induced The absorbance A is the parameter that is usually de-
plasmas can be optimized to yield a detection limit of tens termined with an AAS instrument, where:
of µg/g for certain elements in ideal matrices.
A =−log I/I 0 = log 1/T = k b log e = 0.434k b (5)
The constant k prime is proportional to a number of pa-
IV. ATOMIC ABSORPTION rameters, including the number of atoms per unit volume,
SPECTROMETRY the Einstein transition probability for the absorption pro-
cess, and the energy difference (Fig. 1) between levels 0
A. Theoretical Background and 1. The Beer-Lambert law combines these constants
into one constant a to yield the following equation:
Figure 1B illustrates the basic principle of atomic absorp-
tion spectrometry (AAS). The amount of light absorbed A = abc (6)