Page 153 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 153
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 193
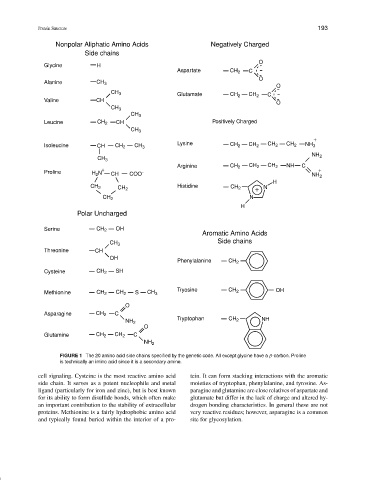
Nonpolar Aliphatic Amino Acids Negatively Charged
Side chains
O
Glycine H
Aspartate CH 2 C
O
Alanine CH 3
O
CH 3 Glutamate CH 2 CH 2 C
Valine CH
O
CH 3
CH 3
Leucine CH 2 CH Positively Charged
CH 3
+
Isoleucine CH CH 2 CH 3 Lysine CH 2 CH 2 CH 2 CH 2 NH 3
NH 2
CH 3
Arginine CH 2 CH 2 CH 2 NH C
+ +
Proline H 2 N CH COO - NH 2
H
CH 2 CH 2 Histidine CH 2 + N
CH 2 N
H
Polar Uncharged
Serine CH 2 OH
Aromatic Amino Acids
CH 3 Side chains
Threonine CH
OH
Phenylalanine CH 2
Cysteine CH 2 SH
Methionine CH 2 CH 2 S CH 3 Tryosine CH 2 OH
O
Asparagine CH 2 C
NH 2 Tryptophan CH 2 NH
O
Glutamine CH 2 CH 2 C
NH 2
FIGURE 1 The 20 amino acid side chains specified by the genetic code. All except glycine have a β-carbon. Proline
is technically an imino acid since it is a secondary amine.
cell signaling. Cysteine is the most reactive amino acid tein. It can form stacking interactions with the aromatic
side chain. It serves as a potent nucleophile and metal moieties of tryptophan, phenylalanine, and tyrosine. As-
ligand (particularly for iron and zinc), but is best known paragine and glutamine are close relatives of aspartate and
for its ability to form disulfide bonds, which often make glutamate but differ in the lack of charge and altered hy-
an important contribution to the stability of extracellular drogen bonding characteristics. In general these are not
proteins. Methionine is a fairly hydrophobic amino acid very reactive residues; however, asparagine is a common
and typically found buried within the interior of a pro- site for glycosylation.