Page 157 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 157
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 197
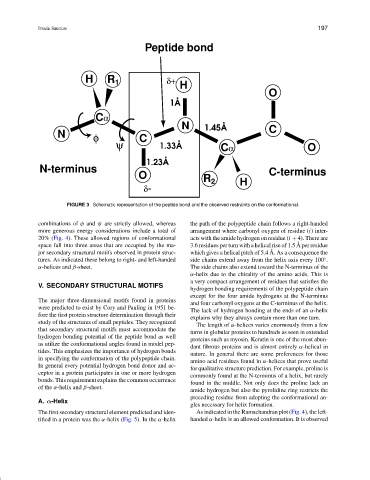
Peptide bond
H R 1 δ+ H
O
1Å
Cα
N 1.45Å C
N φ C
ψ 1.33Å Cα O
1.23Å
N-terminus C-terminus
O R 2 H
δ-
FIGURE 3 Schematic representation of the peptide bond and the observed restraints on the conformational.
combinations of φ and ψ are strictly allowed, whereas the path of the polypeptide chain follows a right-handed
more generous energy considerations include a total of arrangement where carbonyl oxygen of residue (i) inter-
20% (Fig. 4). These allowed regions of conformational acts with the amide hydrogen on residue (i + 4). There are
˚
space fall into three areas that are occupied by the ma- 3.6 residues per turn with a helical rise of 1.5 A per residue
˚
jor secondary structural motifs observed in protein struc- which gives a helical pitch of 5.4 A. As a consequence the
tures. As indicated these belong to right- and left-handed side chains extend away from the helix axis every 100 .
◦
α-helices and β-sheet. The side chains also extend toward the N-terminus of the
α-helix due to the chirality of the amino acids. This is
a very compact arrangement of residues that satisfies the
V. SECONDARY STRUCTURAL MOTIFS
hydrogen bonding requirements of the polypeptide chain
except for the four amide hydrogens at the N-terminus
The major three-dimensional motifs found in proteins
and four carbonyl oxygens at the C-terminus of the helix.
were predicted to exist by Cory and Pauling in 1951 be-
The lack of hydrogen bonding at the ends of an α-helix
fore the first protein structure determination through their
explains why they always contain more than one turn.
study of the structures of small peptides. They recognized
The length of α-helices varies enormously from a few
that secondary structural motifs must accommodate the
turns in globular proteins to hundreds as seen in extended
hydrogen bonding potential of the peptide bond as well
proteins such as myosin. Keratin is one of the most abun-
as utilize the conformational angles found in model pep-
dant fibrous proteins and is almost entirely α-helical in
tides. This emphasizes the importance of hydrogen bonds
nature. In general there are some preferences for those
in specifying the conformation of the polypeptide chain.
amino acid residues found in α-helices that prove useful
In general every potential hydrogen bond donor and ac-
for qualitative structure prediction. For example, proline is
ceptor in a protein participates in one or more hydrogen
commonly found at the N-terminus of a helix, but rarely
bonds. This requirement explains the common occurrence
found in the middle. Not only does the proline lack an
of the α-helix and β-sheet.
amide hydrogen but also the pyrolidine ring restricts the
preceding residue from adopting the conformational an-
A. α-Helix
gles necessary for helix formation.
The first secondary structural element predicted and iden- As indicated in the Ramachandran plot (Fig. 4), the left-
tified in a protein was the α-helix (Fig. 5). In the α-helix handed α-helix is an allowed conformation. It is observed