Page 161 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 161
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 201
(a)
(b)
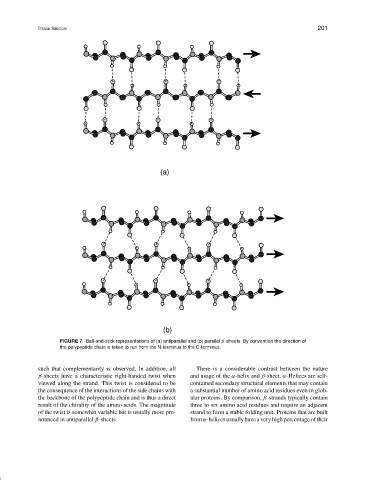
FIGURE 7 Ball-and-stick representations of (a) antiparallel and (b) parallel β-sheets. By convention the direction of
the polypeptide chain is taken to run from the N-terminus to the C-terminus.
such that complementarity is observed. In addition, all There is a considerable contrast between the nature
β-sheets have a characteristic right-handed twist when and usage of the α-helix and β-sheet. α-Helices are self-
viewed along the strand. This twist is considered to be contained secondary structural elements that may contain
the consequence of the interactions of the side chains with a substantial number of amino acid residues even in glob-
the backbone of the polypeptide chain and is thus a direct ular proteins. By comparison, β-strands typically contain
result of the chirality of the amino acids. The magnitude three to six amino acid residues and require an adjacent
of the twist is somewhat variable but is usually more pro- strand to form a stable folding unit. Proteins that are built
nounced in antiparallel β-sheets. from α-helices usually have a very high percentage of their