Page 159 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 159
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 199
O
C
C α
C
C N O
C α
C
O
C α
O C
N C
C α
C
N C
C α
C
C o
N O 5.4 A
C α
C
O C
N
C
O C
N C
C α
C O
C
C C
N
O
C α
C
N C
C α
N
C
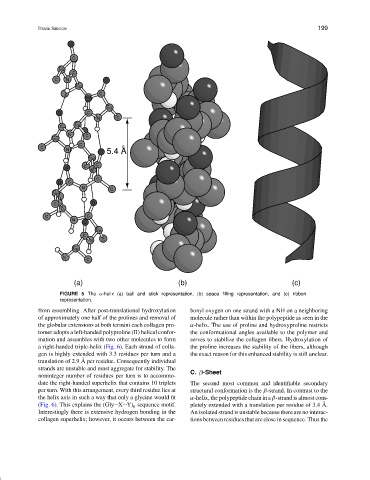
(a) (b) (c)
FIGURE 5 The α-helix (a) ball and stick representation, (b) space filling representation, and (c) ribbon
representation.
from assembling. After post-translational hydroxylation bonyl oxygen on one strand with a NH on a neighboring
of approximately one half of the prolines and removal of molecule rather than within the polypeptide as seen in the
the globular extensions at both termini each collagen pro- α-helix. The use of proline and hydroxyproline restricts
tomer adopts a left-handed polyproline (II) helical confor- the conformational angles available to the polymer and
mation and assembles with two other molecules to form serves to stabilize the collagen fibers. Hydroxylation of
a right-handed triple-helix (Fig. 6). Each strand of colla- the proline increases the stability of the fibers, although
gen is highly extended with 3.3 residues per turn and a the exact reason for this enhanced stability is still unclear.
˚
translation of 2.9 A per residue. Consequently individual
strands are unstable and must aggregate for stability. The
C. β-Sheet
noninteger number of residues per turn is to accommo-
date the right-handed superhelix that contains 10 triplets The second most common and identifiable secondary
per turn. With this arrangement, every third residue lies at structural conformation is the β-strand. In contrast to the
the helix axis in such a way that only a glycine would fit α-helix, the polypeptide chain in a β-strand is almost com-
˚
(Fig. 6). This explains the (Gly X Y) n sequence motif. pletely extended with a translation per residue of 3.4 A.
Interestingly there is extensive hydrogen bonding in the An isolated strand is unstable because there are no interac-
collagen superhelix; however, it occurs between the car- tions between residues that are close in sequence. Thus the