Page 154 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 154
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
194 Protein Structure
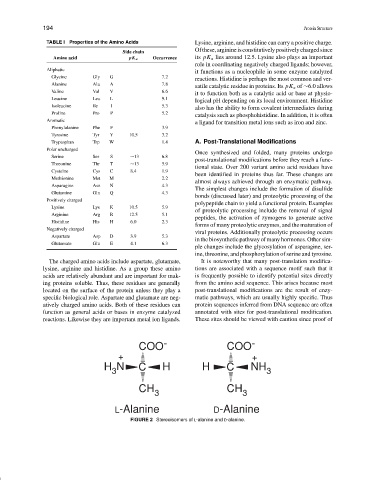
TABLE I Properties of the Amino Acids Lysine, arginine, and histidine can carry a positive charge.
Ofthese,arginineisconstitutivelypositivelychargedsince
Side chain
Amino acid pK a Occurrence its pK a lies around 12.5. Lysine also plays an important
role in coordinating negatively charged ligands; however,
Aliphatic
it functions as a nucleophile in some enzyme catalyzed
Glycine Gly G 7.2
reactions. Histidine is perhaps the most common and ver-
Alanine Ala A 7.8
satile catalytic residue in proteins. Its pK a of ∼6.0 allows
Valine Val V 6.6
it to function both as a catalytic acid or base at physio-
Leucine Leu L 9.1
logical pH depending on its local environment. Histidine
Isoleucine Ile I 5.3
also has the ability to form covalent intermediates during
Proline Pro P 5.2
catalysis such as phosphohistidine. In addition, it is often
Aromatic
a ligand for transition metal ions such as iron and zinc.
Phenylalanine Phe F 3.9
Tyrosine Tyr Y 10.5 3.2
Tryptophan Trp W 1.4 A. Post-Translational Modifications
Polar uncharged
Once synthesized and folded, many proteins undergo
Serine Ser S ∼13 6.8
post-translational modifications before they reach a func-
Threonine Thr T ∼13 5.9
tional state. Over 200 variant amino acid residues have
Cysteine Cys C 8.4 1.9
been identified in proteins thus far. These changes are
Methionine Met M 2.2
almost always achieved through an enzymatic pathway.
Asparagine Asn N 4.3
The simplest changes include the formation of disulfide
Glutamine Gln Q 4.3
bonds (discussed later) and proteolytic processing of the
Positively charged
polypeptide chain to yield a functional protein. Examples
Lysine Lys K 10.5 5.9
of proteolytic processing include the removal of signal
Arginine Arg R 12.5 5.1
peptides, the activation of zymogens to generate active
Histidine His H 6.0 2.3
forms of many proteolytic enzymes, and the maturation of
Negatively charged
viral proteins. Additionally proteolytic processing occurs
Aspartate Asp D 3.9 5.3
in the biosynthetic pathway of many hormones. Other sim-
Glutamate Glu E 4.1 6.3
ple changes include the glycosylation of asparagine, ser-
ine, threonine, and phosphorylation of serine and tyrosine.
The charged amino acids include aspartate, glutamate, It is noteworthy that many post-translation modifica-
lysine, arginine and histidine. As a group these amino tions are associated with a sequence motif such that it
acids are relatively abundant and are important for mak- is frequently possible to identify potential sites directly
ing proteins soluble. Thus, these residues are generally from the amino acid sequence. This arises because most
located on the surface of the protein unless they play a post-translational modifications are the result of enzy-
specific biological role. Aspartate and glutamate are neg- matic pathways, which are usually highly specific. Thus
atively charged amino acids. Both of these residues can protein sequences inferred from DNA sequence are often
function as general acids or bases in enzyme catalyzed annotated with sites for post-translational modification.
reactions. Likewise they are important metal ion ligands. These sites should be viewed with caution since proof of
COO - COO -
+ +
H N C H H C NH
3 3
CH CH
3 3
L-Alanine D-Alanine
FIGURE 2 Stereoisomers of L-alanine and D-alanine.