Page 173 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 173
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 213
Papain
(9PAP)
Ubiquitin
(1UBQ) (b)
(a)
Glutamine Prpp Amidotransferase
Ribonuclease N-terminal domain
(7RSA) (1ECC)
(c) (d)
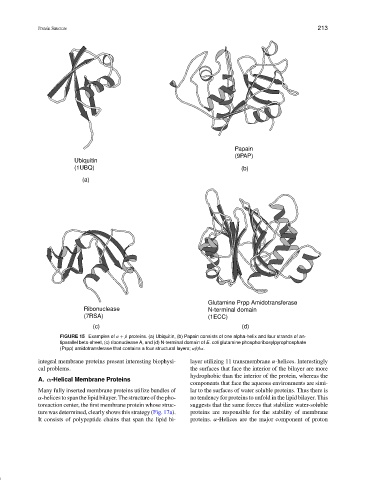
FIGURE 15 Examples of α + β proteins. (a) Ubiquitin, (b) Papain consists of one alpha-helix and four strands of an-
tiparallel beta-sheet, (c) ribonuclease A, and (d) N-terminal domain of E. coli glutamine phosphoribosylpyrophosphate
(Prpp) amidotransferase that contains a four structural layers; αββα.
integral membrane proteins present interesting biophysi- layer utilizing 11 transmembrane α-helices. Interestingly
cal problems. the surfaces that face the interior of the bilayer are more
hydrophobic than the interior of the protein, whereas the
A. α-Helical Membrane Proteins
components that face the aqueous environments are simi-
Many fully inserted membrane proteins utilize bundles of lar to the surfaces of water soluble proteins. Thus there is
α-helicestospanthelipidbilayer.Thestructureofthepho- no tendency for proteins to unfold in the lipid bilayer. This
toreaction center, the first membrane protein whose struc- suggests that the same forces that stabilize water-soluble
ture was determined, clearly shows this strategy (Fig. 17a). proteins are responsible for the stability of membrane
It consists of polypeptide chains that span the lipid bi- proteins. α-Helices are the major component of proton