Page 54 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 54
P1: GTQ Final
Encyclopedia of Physical Science and Technology EN006P-81 June 29, 2001 21:48
838 Glycoconjugates and Carbohydrates
H O CH 2 OH
1 C H O H
2 H
H C OH OH H
3 HO OMe
HO C H D-Glucose.
4 H OH
H C OH
Methyl α-D-glucopyranoside
5
H C OH
6
CH 2 OH CH 2 OH
H O OMe
H
6
CH 2 OH OH H
5 HO H
C OH
H H
4 H H OH
C C Methyl β -D-glucopyranoside
OH H
O
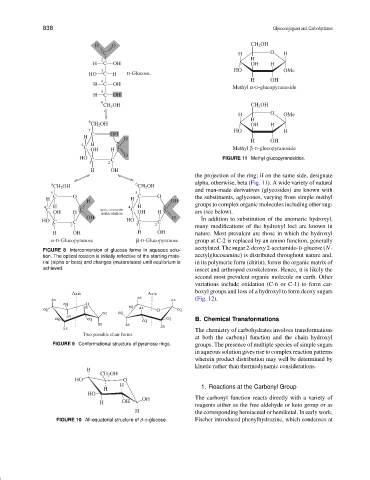
HO C C FIGURE 11 Methyl glucopyranosides.
3 2
H OH
the projection of the ring; if on the same side, designate
6 6 alpha, otherwise, beta (Fig. 11). A wide variety of natural
CH 2 OH CH 2 OH
and man-made derivatives (glycosides) are known with
5 5
C O C O the substituents, aglycones, varying from simple methyl
H H H OH
4 H 4 H groups to complex organic molecules including other sug-
C C C C
OH H mutarotation OH H ars (see below).
OH H In addition to substitution of the anomeric hydroxyl,
HO C C HO C C
3 2 3 2 many modifications of the hydroxyl loci are known in
H OH H OH nature. Most prevalent are those in which the hydroxyl
-D-Glucopyranose -D-Glucopyranose group at C-2 is replaced by an amino function, generally
acetylated.Thesugar2-deoxy2-acetamido-D-glucose(N-
FIGURE 8 Interconversion of glucose forms in aqueous solu-
tion. The optical rotation is initially reflective of the starting mate- acetylglucosamine) is distributed throughout nature and,
rial (alpha or beta) and changes (mutarotates) until equlibrium is in its polymeric form (chitin), forms the organic matrix of
achieved. insect and arthropod exoskeletons. Hence, it is likely the
second most prevalent organic molecule on earth. Other
variations include oxidation (C-6 or C-1) to form car-
boxyl groups and loss of a hydroxyl to form deoxy sugars
Axis Axis
ax
ax ax (Fig. 12).
eq O
eq ax eq ax O eq
eq eq
ax
eq eq eq eq B. Chemical Transformations
ax ax
ax ax
The chemistry of carbohydrates involves transformations
Two possible chair forms
at both the carbonyl function and the chain hydroxyl
FIGURE 9 Conformational structure of pyranose rings. groups. The presence of multiple species of simple sugars
in aqueous solution gives rise to complex reaction patterns
wherein product distribution may well be determined by
kinetic rather than thermodynamic considerations.
H
CH 2 OH
HO O
H 1. Reactions at the Carbonyl Group
H
HO
OH The carbonyl function reacts directly with a variety of
H OH
reagents either as the free aldehyde or keto group or as
H the corresponding hemiacetal or hemiketal. In early work,
FIGURE 10 All-equatorial structure of β-D-glucose. Fischer introduced phenylhydrazine, which condenses at