Page 95 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 95
P1: GLM/GLT P2: FQP Final Pages
Encyclopedia of Physical Science and Technology En004F-171 June 8, 2001 17:11
362 Dielectric Gases
V s of the constituent component gases. In Fig. 4b, exam-
ples are given of binary mixtures of a buffer gas that slows
down electrons efficiently (CO, N 2 , or CO 2 ) and the elec-
tronegative gas SF 6 . At all gas compositions, the measured
(V s ) mix exceedsthepartial-pressure-weightedvaluesofthe
individual components. This has been referred to as syn-
ergism. Similar results are shown in Fig. 4c for mixtures
of the strongly electronegative gas c-C 4 F 8 and the nonpo-
lar weakly electron-attaching buffer gas CF 4 or the polar
buffer gases CHF 3 or 1,1,1-CH 3 CF 3 , which slow down
electrons efficiently via dipole scattering. The (V s ) mix for
the polar gas-containing mixtures far exceeds the partial-
pressure-weighted V s , especially for small percentages of
the electronegative gases.
In Fig. 4c the behavior of curve 1 is interesting in
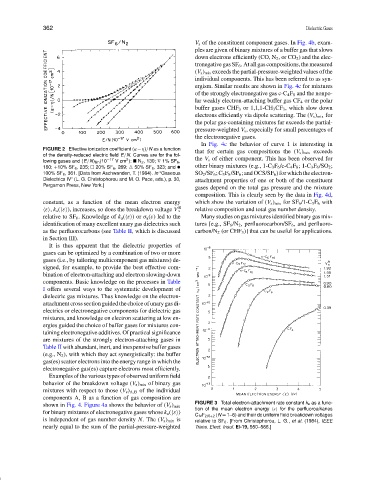
FIGURE 2 Effective ionization coefficient (α −η)/N as a function
that for certain gas compositions the (V s ) mix exceeds
of the density-reduced electric field E /N. Curves are for the fol-
2
lowing gases and (E /N) lim (10 −17 V cm ): N 2 , 130; ∇ 1% SF 6 , the V s of either component. This has been observed for
other binary mixtures (e.g., 1-C 3 F 6 /c-C 4 F 8 ; 1-C 3 F 6 /SO 2 ;
160; +10% SF 6 , 235; 20% SF 6 , 269; 50% SF 6 , 323; and •
100% SF 6 , 361. [Data from Aschwanden, T. (1984). In “Gaseous SO 2 /SF 6 ; C 3 F 8 /SF 6 ; and OCS/SF 6 ) for which the electron-
Dielectrics IV” (L. G. Christophorou and M. O. Pace, eds.), p. 30, attachment properties of one or both of the constituent
Pergamon Press, New York.]
gases depend on the total gas pressure and the mixture
composition. This is clearly seen by the data in Fig. 4d,
constant, as a function of the mean electron energy which show the variation of (V s ) mix for SF 6 /1-C 3 F 6 with
R
ε , k a ( ε ), increases, so does the breakdown voltage V s relative composition and total gas number density.
relative to SF 6 . Knowledge of k a ( ε ) or σ a (ε) led to the Many studies on gas mixtures identified binary gas mix-
identification of many excellent unary gas dielectrics such tures [e.g., SF 6 /N 2 , perfluorocarbon/SF 6 , and perfluoro-
as the perfluorocarbons (see Table II, which is discussed carbon/N 2 (or CHF 3 )] that can be useful for applications.
in Section III).
It is thus apparent that the dielectric properties of
gases can be optimized by a combination of two or more
gases (i.e., by tailoring multicomponent gas mixtures) de-
signed, for example, to provide the best effective com-
bination of electron-attaching and electron slowing-down
components. Basic knowledge on the processes in Table
I offers several ways to the systematic development of
dielectric gas mixtures. Thus knowledge on the electron-
attachment cross section guided the choice of unary gas di-
electrics or electronegative components for dielectric gas
mixtures, and knowledge on electron scattering at low en-
ergies guided the choice of buffer gases for mixtures con-
taining electronegative additives. Of practical significance
are mixtures of the strongly electron-attaching gases in
Table II with abundant, inert, and inexpensive buffer gases
(e.g., N 2 ), with which they act synergistically: the buffer
gas(es) scatter electrons into the energy range in which the
electronegative gas(es) capture electrons most efficiently.
Examples of the various types of observed uniform field
behavior of the breakdown voltage (V s ) mix of binary gas
mixtures with respect to those (V s ) A,B of the individual
components A, B as a function of gas composition are
FIGURE 3 Total electron-attachment rate constant k a as a func-
shown in Fig. 4. Figure 4a shows the behavior of (V s ) mix
tion of the mean electron energy ε for the perfluoroalkanes
for binary mixtures of electronegative gases whose k a ( ε )
C N F 2N+2 (N = 1–6) and their dc uniform field breakdown voltages
is independent of gas number density N. The (V s ) mix is relative to SF 6 . [From Christophorou, L. G., et al. (1984), IEEE
nearly equal to the sum of the partial-pressure-weighted Trans. Elect. Insul. El-19, 550–566.]