Page 96 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 96
P1: GLM/GLT P2: FQP Final Pages
Encyclopedia of Physical Science and Technology En004F-171 June 8, 2001 17:11
Dielectric Gases 363
R
dc uniform field breakdown strengths V s in Table II,
which were measured at approximately atmospheric pres-
sures: The V s of air is ∼50 times higher than that of
Ne, and the V s of SF 6 is ∼3.3 times higher than that
of air. The highest known V s (∼2.5 times higher than
SF 6 ) are exhibited by strongly electron-attaching poly-
atomic gases such as the perfluorocarbons in Table II
and other polyhalogenated molecules. Weakly electron-
attaching or non-electron-attaching gases (Table II) have
low V s values. Nonelectronegative molecular gases with
large electron-scattering cross sections have reasonably
high V s values compared, for instance, with the rare
gases, in which low-energy electron scattering is totally
elastic.
The V s of a gaseous medium is expected, in accordance
with Paschen’s law, to be a function only of Nd s (the
product of the gas number density N and the electrode
separation d s ); thus, for sufficiently high values of N to
the right of the Paschen minimum, V s /Nd s = (E/N) lim
should be independent of N. This relationship holds for
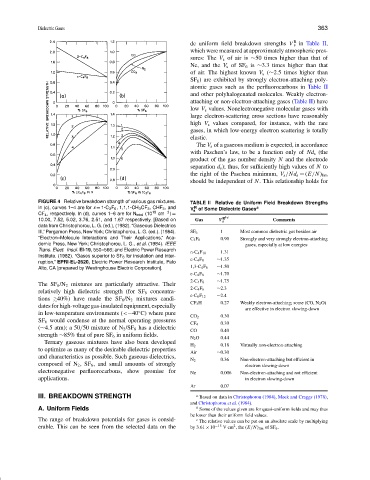
FIGURE 4 Relative breakdown strangth of various gas mixtures. TABLE II Relative dc Uniform Field Breakdown Strengths
In (c), curves 1–4 are for x = 1-C 3 F 6 , 1,1,1-CH 3 CF 3 , CHF 3 , and V of Some Dielectric Gases a
R
s
CF 4 , respectively. In (d), curves 1–6 are for N total (10 19 cm −3 ) =
10.00, 7.52, 5.02, 3.76, 2.51, and 1.67 respectively. [Based on Gas V R b,c Comments
s
data from Christophorou, L. G. (ed.), (1982). “Gaseous Dielectrics
III,” Pergamon Press, New York; Christophorou, L. G. (ed.). (1984). SF 6 1 Most common dielectric gas besides air
“Electron–Molecule Interactions and Their Applications,” Aca- C 3 F 8 0.90 Strongly and very strongly electron-attaching
demic Press, New York; Christophorou, L. G., et al. (1984). IEEE gases, especially at low energies
Trans. Elect. Insul. El-19, 550–566; and Electric Power Research
n-C 4 F 10 1.31
Institute. (1982). “Gases superior to SF 6 for insulation and inter-
c-C 4 F 8 ∼1.35
ruption,” EPRI·EL-2620, Electric Power Research Institute, Palo
Alto, CA [prepared by Westinghouse Electric Corporation]. 1,3-C 4 F 6 ∼1.50
c-C 4 F 6 ∼1.70
2-C 4 F 8 ∼1.75
The SF 6 /N 2 mixtures are particularly attractive. Their
2-C 4 F 6 ∼2.3
relatively high dielectric strength (for SF 6 concentra-
c-C 6 F 12 ∼2.4
tions 40%) have made the SF 6 /N 2 mixtures candi-
CF 3 H 0.27 Weakly electron-attaching; some (CO, N 2 O)
dates for high-voltage gas-insulated equipment, especially
are effective in electron slowing-down
in low-temperature environments (<−40 C) where pure
◦
CO 2 0.30
SF 6 would condense at the normal operating pressures
CF 4 0.39
(∼4.5 atm); a 50/50 mixture of N 2 /SF 6 has a dielectric
CO 0.40
strength ∼85% that of pure SF 6 in uniform fields.
N 2 O 0.44
Ternary gaseous mixtures have also been developed
H 2 0.18 Virtually non-electron-attaching
to optimize as many of the desirable dielectric properties
Air ∼0.30
and characteristics as possible. Such gaseous dielectrics,
N 2 0.36 Non-electron-attaching but efficient in
composed of N 2 , SF 6 , and small amounts of strongly electron slowing-down
electronegative perfluorocarbons, show promise for Ne 0.006 Non-electron-attaching and not efficient
applications. in electron slowing-down
Ar 0.07
III. BREAKDOWN STRENGTH a Based on data in Christophorou (1984), Meek and Craggs (1978),
and Christophorou et al. (1984).
A. Uniform Fields b Some of the values given are for quasi-uniform fields and may thus
be lower than their uniform field values.
The range of breakdown potentials for gases is consid- c The relative values can be put on an absolute scale by multiplying
erable. This can be seen from the selected data on the by 3.61 × 10 −15 Vcm , the (E/N) lim of SF 6 .
2