Page 19 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 19
14 I / AFFINITY SEPARATION/ Derivatization
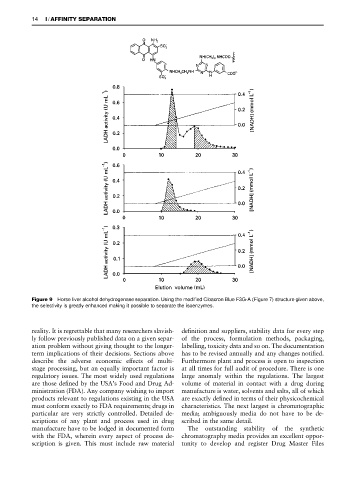
Figure 9 Horse liver alcohol dehydrogenase separation. Using the modified Cibacron Blue F3G-A (Figure 7) structure given above,
the selectivity is greatly enhanced making it possible to separate the isoenzymes.
reality. It is regrettable that many researchers slavish- deRnition and suppliers, stability data for every step
ly follow previously published data on a given separ- of the process, formulation methods, packaging,
ation problem without giving thought to the longer- labelling, toxicity data and so on. The documentation
term implications of their decisions. Sections above has to be revised annually and any changes notiRed.
describe the adverse economic effects of multi- Furthermore plant and process is open to inspection
stage processing, but an equally important factor is at all times for full audit of procedure. There is one
regulatory issues. The most widely used regulations large anomaly within the regulations. The largest
are those deRned by the USA’s Food and Drug Ad- volume of material in contact with a drug during
ministration (FDA). Any company wishing to import manufacture is water, solvents and salts, all of which
products relevant to regulations existing in the USA are exactly deRned in terms of their physicochemical
must conform exactly to FDA requirements; drugs in characteristics. The next largest is chromatographic
particular are very strictly controlled. Detailed de- media; ambiguously media do not have to be de-
scriptions of any plant and process used in drug scribed in the same detail.
manufacture have to be lodged in documented form The outstanding stability of the synthetic
with the FDA, wherein every aspect of process de- chromatography media provides an excellent oppor-
scription is given. This must include raw material tunity to develop and register Drug Master Files