Page 16 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 16
Sepsci*1*TSK*Venkatachala=BG
I / AFFINITY SEPARATION 11
The Rnal step is to design appropriate bonding purities, substantial increases in column lifetime, and
technologies to minimize potential leakage. Until re- improvements in batch-to-batch reproducibility.
cently this type of modelling was a purely theoretical
exercise. It was only the introduction of computer-
assisted molecular modelling techniques that allowed Rational Design of Af\nity Ligands
the theory to be tested. Before the arrival of logical
modellling the discovery of selective ligands was en- Modi\cation of Existing Structures
tirely based upon empirical observation, later fol- The Rrst example of a rational design of new bio-
lowed by a combination of observation, experience mimetic dyes used the interaction between horse
and limited assistance from early computer generated liver alcohol dehydrogenase (ADH) and analogues of
models. Although several novel structures evolved the textile dye Cibacron Blue F3G-A (Figure 7). It
during this period, a general approach to the design of had been established that the parent dye binds in the
#
new structures remained elusive. At this time only NAD -binding site of the enzyme, with the an-
very few 3-D protein structures were available, again thraquinone, diaminobenzene sulfonate and triazine
greatly restricting application of rational design ap- rings (rings A, B and C, respectively, in Figure 7)
proaches. As more sophisticated programmes, simu- apparently adopting similar positions to those of the
lation techniques, protein fragment data and many adenine adenosine ribose and pyrophosphate groups
more protein structures were released, logical design of NAD . The anthraquinone ring (A) binds in
methods were revolutionized. However, many mil- a wide apolar fold that constitutes, at one end, the
lions of proteins are involved in life processes, and it adenine bridging site, while the bridging ring (B) is
is clear that many years will elapse before the major- positioned such that its sulfonate group interacts with
ity of these will be fully described by accurate models. the guanidinium side chain of Arg271 (Figure 8).
Consequently intuition and experience will continue Ring C binds close to where the pyrophosphate
to play a major role in the design of suitable ligands. bridge of the coenzyme binds with the reactive
Of available rationally designed synthetic molecules, triazinyl chlorine adjacent to the nicotinamide ribose-
the Mimetic range can currently separate over 50% binding site. The terminal ring (D) appears to be
of a randomly selected range of proteins. Stability bound in a fold between the catalytic and coenzyme
under depyrogenating conditions has been demon- binding domains, with a possible interaction of the
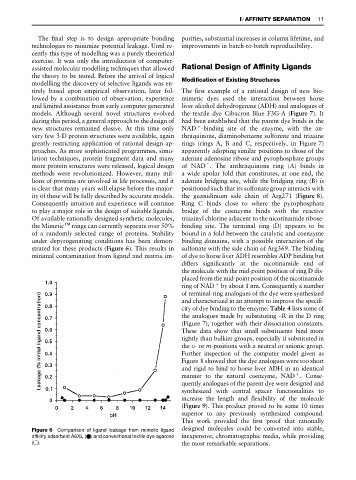
strated for these products (Figure 6). This results in sulfonate with the side chain of Arg369. The binding
minimal contamination from ligand and matrix im- of dye to horse liver ADH resembles ADP binding but
differs signiRcantly at the nicotinamide end of
the molecule with the mid-point position of ring D dis-
placed from the mid-point position of the nicotinamide
ring of NAD # by about 1 nm. Consequently a number
of terminal-ring analogues of the dye were synthesized
and characterized in an attempt to improve the speciR-
city of dye binding to the enzyme. Table 4 lists some of
the analogues made by substituting }Rin the D ring
(Figure 7), together with their dissociation constants.
These data show that small substituents bind more
tightly than bulkier groups, especially if substituted in
the o-or m-positions with a neutral or anionic group.
Further inspection of the computer model given as
Figure 8 showed that the dye analogues were too short
andrigid to bind to horseliver ADHinanidentical
#
manner to the natural coenzyme, NAD .Conse-
quently analogues of the parent dye were designed and
synthesized with central spacer functionalities to
increase the length and Sexibility of the molecule
(Figure 9). This product proved to be some 10 times
superior to any previously synthesized compound.
This work provided the Rrst proof that rationally
Figure 6 Comparison of ligand leakage from mimetic ligand designed molecules could be converted into stable,
affinity adsorbent A6XL ( ) and conventional textile dye agarose inexpensive, chromatographic media, while providing
( ). the most remarkable separations.