Page 14 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 14
Sepsci*1*TSK*Venkatachala=BG
I / AFFINITY SEPARATION 9
ity. If stable, highly selective and inexpensive af- a simple synthesized entity or a high molecular weight
Rnity ligands were available, then opportunities would protein. The afRnity technique is theoretically of
exist for researchers to develop efRcient high yield universal application and any protein can be separ-
separations even in the earliest phases of investigation. ated whatever its structure and origin. As always,
These systems could then be passed forward to pro- there are major limitations. The most effective
duction with the knowledge that optimally efR- afRnity ligands are other proteins. Unfortunately
cient separations are immediately achievable. such proteins are difRcult to Rnd, identify, isolate
Production costs of any pure material reSect the and purify. This results in high costs. An even greater
absolute purity level required and the difRculty of deterrent is that most proteins are chemically, cata-
achieving it. Therapeutic proteins have high purity lytically and enzymically unstable, a particularly un-
requirements and the larger the administered dose, attractive feature if they are to be used for the manu-
the purer it has to be. Since many protein pharma- facture of therapeutic substances; and regulatory
ceuticals will be used at high dose levels, purities need authorities generally reject applications using pro-
to exceed 99%, occasionally up to 99.999%. That teinaceous ligands.
these purities can be met by traditional methods is In anticipating that one day stable inexpensive af-
possible, but it is widely documented that the applica- Rnity media would be in demand, a team led by C.R.
tion of such methods massively increases production Lowe began an investigation into which synthetic
costs. Between 50 and 80% of total production costs ligand structures offered the greatest possibility
of therapeutic proteins are incurred at the puriRcation of developing inexpensive stable ligands. It was con-
stage. The manufacturing cost of a product is directly cluded that structures that could be manipulated into
related to its concentration in the mother liquor; speciRc spatial geometries and to which intermolecu-
the more dilute it is, the higher the cost of recov- lar binding forces could easily be added offered
ery. Since traditional puriRcation processes on their the highest chance of success. Model compounds
own cannot selectively concentrate a target protein to were already available; the textile dyes.
the exclusion of all others, they have to be used in
series. The number of stages required can vary be- Synthetic Ligands
tween four and 15. Each step represents a yield loss,
and incurs a processing cost. Yields of less than 20% Textile dyes had already proved to be suitable ligands
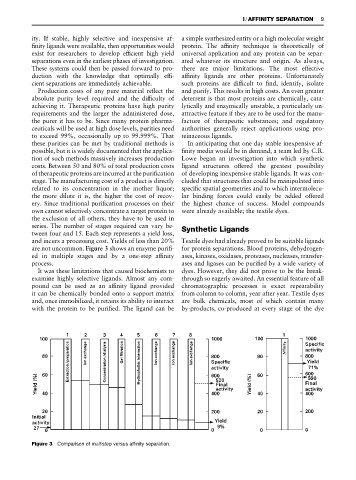
are not uncommon. Figure 3 shows an enzyme puriR- for protein separations. Blood proteins, dehydrogen-
ed in multiple stages and by a one-step afRnity ases, kinases, oxidases, proteases, nucleases, transfer-
process. ases and ligases can be puriRed by a wide variety of
It was these limitations that caused biochemists to dyes. However, they did not prove to be the break-
examine highly selective ligands. Almost any com- through so eagerly awaited. An essential feature of all
pound can be used as an afRnity ligand provided chromatographic processes is exact repeatability
it can be chemically bonded onto a support matrix from column to column, year after year. Textile dyes
and, once immobilized, it retains its ability to interact are bulk chemicals, most of which contain many
with the protein to be puriRed. The ligand can be by-products, co-produced at every stage of the dye
Figure 3 Comparison of multistep versus affinity separation.