Page 15 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 15
10 I / AFFINITY SEPARATION/ Derivatization
manufacturing process. This fact alone makes repro-
ducibility problematic. Furthermore, the bonding
process between dye and matrix was poorly re-
searched. This resulted in extensive leakage. All com-
mercially available textile dye products leak exten-
sively, especially under depyrogenating conditions
(Figure 4). Despite these limitations, it was recog-
nized that dye-like structures had a powerful underly-
ing ability to separate a very diverse range of proteins.
Their relatively complex chemical structures allow
spatial manipulation of their basic skeletons into
an inRnite variety of shapes and conRgurations. Pro-
teins are complex three-dimensional (3-D) structures
and folds are present throughout all protein struc-
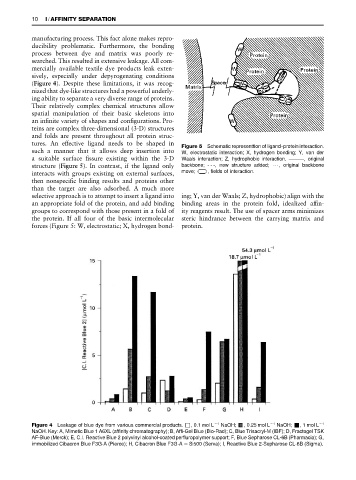
tures. An effective ligand needs to be shaped in Figure 5 Schematic representtion of ligand}protein interaction.
such a manner that it allows deep insertion into W, electrostatic interaction; X, hydrogen bonding; Y, van der
a suitable surface Rssure existing within the 3-D Waals interaction; Z, hydrophobic interaction. ***, original
structure (Figure 5). In contrast, if the ligand only backbone; - - -, new structure added; 2, original backbone
interacts with groups existing on external surfaces, move; , fields of interaction.
then nonspeciRc binding results and proteins other
than the target are also adsorbed. A much more
selective approach is to attempt to insert a ligand into ing; Y, van der Waals; Z, hydrophobic) align with the
an appropriate fold of the protein, and add binding binding areas in the protein fold, idealized afRn-
groups to correspond with those present in a fold of ity reagents result. The use of spacer arms minimizes
the protein. If all four of the basic intermolecular steric hindrance between the carrying matrix and
forces (Figure 5: W, electrostatic; X, hydrogen bond- protein.
1
1
Figure 4 Leakage of blue dye from various commercial products. , 0.1 mol L NaOH; , 0.25 mol L NaOH; , 1 mol L 1
NaOH. Key: A, Mimetic Blue 1 A6XL (affinity chromatography); B, Affi-Gel Blue (Bio-Rad); C, Blue Trisacryl-M (IBF); D, Fractogel TSK
AF-Blue (Merck); E, C.I. Reactive Blue 2 polyvinyl alcohol-coated perfluropolymer support; F, Blue Sepharose CL-6B (Pharmacia); G,
immobilized Cibacron Blue F3G-A (Pierce); H, Cibacron Blue F3G-A"Si500 (Serva); I, Reactive Blue 2-Sepharose CL-6B (Sigma).