Page 18 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 18
Sepsci*1*TSK*Venkatachala=BG
I / AFFINITY SEPARATION 13
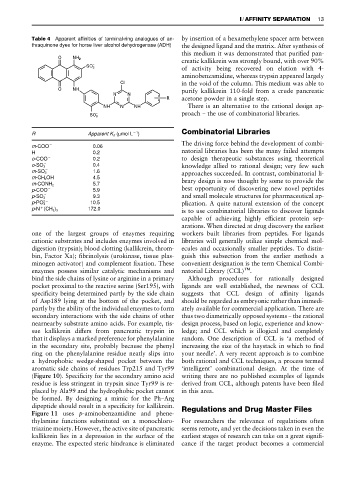
Table 4 Apparent affinities of terminal-ring analogues of an- by insertion of a hexamethylene spacer arm between
thraquinone dyes for horse liver alcohol dehydrogenase (ADH) the designed ligand and the matrix. After synthesis of
this medium it was demonstrated that puriRed pan-
creatic kallikrein was strongly bound, with over 90%
of activity being recovered on elution with 4-
aminobenzamidine, whereas trypsin appeared largely
in the void of the column. This medium was able to
purify kallikrein 110-fold from a crude pancreatic
acetone powder in a single step.
There is an alternative to the rational design ap-
proach } the use of combinatorial libraries.
1
R Apparent K d ( mol L ) Combinatorial Libraries
The driving force behind the development of combi-
m-COO 0.06
H 0.2 natorial libraries has been the many failed attempts
o-COO 0.2 to design therapeutic substances using theoretical
o-SO 0.4 knowledge allied to rational design; very few such
3
m-SO 1.6 approaches succeeded. In contrast, combinatorial li-
3
m-CH 2 OH 4.5
brary design is now thought by some to provide the
5.7
m-CONH 2
p-COO 5.9 best opportunity of discovering new novel peptides
p-SO 9.3 and small molecule structures for pharmaceutical ap-
3
2
p-PO 10.5 plication. A quite natural extension of the concept
3
#
is to use combinatorial libraries to discover ligands
p-N (CH 3 ) 3 172.0
capable of achieving highly efRcient protein sep-
arations. When directed at drug discovery the earliest
one of the largest groups of enzymes requiring workers built libraries from peptides. For ligands
cationic substrates and includes enzymes involved in libraries will generally utilize simple chemical mol-
digestion (trypsin); blood clotting (kallikrein, throm- ecules and occasionally smaller peptides. To distin-
bin, Factor Xa); Rbrinolysis (urokinase, tissue plas- guish this subsection from the earlier methods a
minogen activator) and complement Rxation. These convenient designation is the term Chemical Combi-
enzymes possess similar catalytic mechanisms and natorial Library (CCL) .
bind the side chains of lysine or arginine in a primary Although procedures for rationally designed
pocket proximal to the reactive serine (Ser195), with ligands are well established, the newness of CCL
speciRcity being determined partly by the side chain suggests that CCL design of afRnity ligands
of Asp189 lying at the bottom of the pocket, and should be regarded as embryonic rather than immedi-
partly by the ability of the individual enzymes to form ately available for commercial application. There are
secondary interactions with the side chains of other thus two diametrically opposed systems } the rational
nearnearby substrate amino acids. For example, tis- design process, based on logic, experience and know-
sue kallikrein differs from pancreatic trypsin in ledge; and CCL which is illogical and completely
that it displays a marked preference for phenylalanine random. One description of CCL is ‘a method of
in the secondary site, probably because the phenyl increasing the size of the haystack in which to Rnd
ring on the phenylalanine residue neatly slips into your needle’. A very recent approach is to combine
a hydrophobic wedge-shaped pocket between the both rational and CCL techniques, a process termed
aromatic side chains of residues Trp215 and Tyr99 ‘intelligent’ combinational design. At the time of
(Figure 10). SpeciRcity for the secondary amino acid writing there are no published examples of ligands
residue is less stringent in trypsin since Tyr99 is re- derived from CCL, although patents have been Rled
placed by Ala99 and the hydrophobic pocket cannot in this area.
be formed. By designing a mimic for the Ph}Arg
dipeptide should result in a speciRcity for kallikrein. Regulations and Drug Master Files
Figure 11 uses p-aminobenzamidine and phene-
thylamine functions substituted on a monochloro- For researchers the relevance of regulations often
triazine moiety. However, the active site of pancreatic seems remote, and yet the decisions taken in even the
kallikrein lies in a depression in the surface of the earliest stages of research can take on a great signiR-
enzyme. The expected steric hindrance is eliminated cance if the target product becomes a commercial