Page 46 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 46
Sepsci*21*TSK*Venkatachala=BG
I / CHROMATOGRAPHY 41
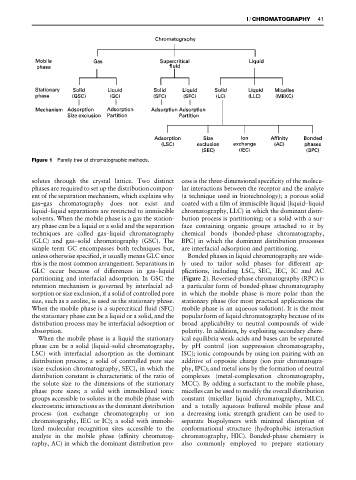
Figure 1 Family tree of chromatographic methods.
solutes through the crystal lattice. Two distinct cess is the three-dimensional speciRcity of the molecu-
phases are required to set up the distribution compon- lar interactions between the receptor and the analyte
ent of the separation mechanism, which explains why (a technique used in biotechnology); a porous solid
gas}gas chromatography does not exist and coated with a Rlm of immiscible liquid (liquid}liquid
liquid}liquid separations are restricted to immiscible chromatography, LLC) in which the dominant distri-
solvents. When the mobile phase is a gas the station- bution process is partitioning; or a solid with a sur-
ary phase can be a liquid or a solid and the separation face containing organic groups attached to it by
techniques are called gas}liquid chromatography chemical bonds (bonded-phase chromatography,
(GLC) and gas}solid chromatography (GSC). The BPC) in which the dominant distribution processes
simple term GC encompasses both techniques but, are interfacial adsorption and partitioning.
unless otherwise speciRed, it usually means GLC since Bonded phases in liquid chromatography are wide-
this is the most common arrangement. Separations in ly used to tailor solid phases for different ap-
GLC occur because of differences in gas}liquid plications, including LSC, SEC, IEC, IC and AC
partitioning and interfacial adsorption. In GSC the (Figure 2). Reversed-phase chromatography (RPC) is
retention mechanism is governed by interfacial ad- a particular form of bonded-phase chromatography
sorption or size exclusion, if a solid of controlled pore in which the mobile phase is more polar than the
size, such as a zeolite, is used as the stationary phase. stationary phase (for most practical applications the
When the mobile phase is a supercritical Suid (SFC) mobile phase is an aqueous solution). It is the most
the stationary phase can be a liquid or a solid, and the popular form of liquid chromatography because of its
distribution process may be interfacial adsorption or broad applicability to neutral compounds of wide
absorption. polarity. In addition, by exploiting secondary chem-
When the mobile phase is a liquid the stationary ical equilibria weak acids and bases can be separated
phase can be a solid (liquid}solid chromatography, by pH control (ion suppression chromatography,
LSC) with interfacial adsorption as the dominant ISC); ionic compounds by using ion pairing with an
distribution process; a solid of controlled pore size additive of opposite charge (ion pair chromatogra-
(size exclusion chromatography, SEC), in which the phy, IPC); and metal ions by the formation of neutral
distribution constant is characteristic of the ratio of complexes (metal-complexation chromatography,
the solute size to the dimensions of the stationary MCC). By adding a surfactant to the mobile phase,
phase pore sizes; a solid with immobilized ionic micelles can be used to modify the overall distribution
groups accessible to solutes in the mobile phase with constant (micellar liquid chromatography, MLC),
electrostatic interactions as the dominant distribution and a totally aqueous buffered mobile phase and
process (ion exchange chromatography or ion a decreasing ionic strength gradient can be used to
chromatography, IEC or IC); a solid with immobi- separate biopolymers with minimal disruption of
lized molecular recognition sites accessible to the conformational structure (hydrophobic interaction
analyte in the mobile phase (afRnity chromatog- chromatography, HIC). Bonded-phase chemistry is
raphy, AC) in which the dominant distribution pro- also commonly employed to prepare stationary