Page 50 - Subyek Encyclopedia - Encyclopedia of Separation Science
P. 50
Sepsci*21*TSK*Venkatachala=BG
I / CHROMATOGRAPHY 45
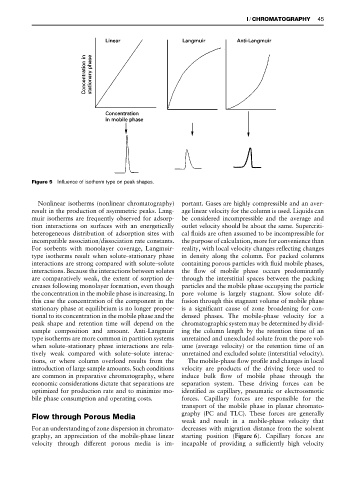
Figure 5 Influence of isotherm type on peak shapes.
Nonlinear isotherms (nonlinear chromatography) portant. Gases are highly compressible and an aver-
result in the production of asymmetric peaks. Lang- age linear velocity for the column is used. Liquids can
muir isotherms are frequently observed for adsorp- be considered incompressible and the average and
tion interactions on surfaces with an energetically outlet velocity should be about the same. Supercriti-
heterogeneous distribution of adsorption sites with cal Suids are often assumed to be incompressible for
incompatible association/dissociation rate constants. the purpose of calculation, more for convenience than
For sorbents with monolayer coverage, Langmuir- reality, with local velocity changes reSecting changes
type isotherms result when solute}stationary phase in density along the column. For packed columns
interactions are strong compared with solute}solute containing porous particles with Suid mobile phases,
interactions. Because the interactions between solutes the Sow of mobile phase occurs predominantly
are comparatively weak, the extent of sorption de- through the interstitial spaces between the packing
creases following monolayer formation, even though particles and the mobile phase occupying the particle
the concentration in the mobile phase is increasing. In pore volume is largely stagnant. Slow solute dif-
this case the concentration of the component in the fusion through this stagnant volume of mobile phase
stationary phase at equilibrium is no longer propor- is a signiRcant cause of zone broadening for con-
tional to its concentration in the mobile phase and the densed phases. The mobile-phase velocity for a
peak shape and retention time will depend on the chromatographic system may be determined by divid-
sample composition and amount. Anti-Langmuir ing the column length by the retention time of an
type isotherms are more common in partition systems unretained and unexcluded solute from the pore vol-
when solute}stationary phase interactions are rela- ume (average velocity) or the retention time of an
tively weak compared with solute}solute interac- unretained and excluded solute (interstitial velocity).
tions, or where column overload results from the The mobile-phase Sow proRle and changes in local
introduction of large sample amounts. Such conditions velocity are products of the driving force used to
are common in preparative chromatography, where induce bulk Sow of mobile phase through the
economic considerations dictate that separations are separation system. These driving forces can be
optimized for production rate and to minimize mo- identiRed as capillary, pneumatic or electroosmotic
bile phase consumption and operating costs. forces. Capillary forces are responsible for the
transport of the mobile phase in planar chromato-
graphy (PC and TLC). These forces are generally
Flow through Porous Media
weak and result in a mobile-phase velocity that
For an understanding of zone dispersion in chromato- decreases with migration distance from the solvent
graphy, an appreciation of the mobile-phase linear starting position (Figure 6). Capillary forces are
velocity through different porous media is im- incapable of providing a sufRciently high velocity