Page 215 - Engineered Interfaces in Fiber Reinforced Composites
P. 215
Chapter 5. Surface treatments offibers and effects on composite properties 197
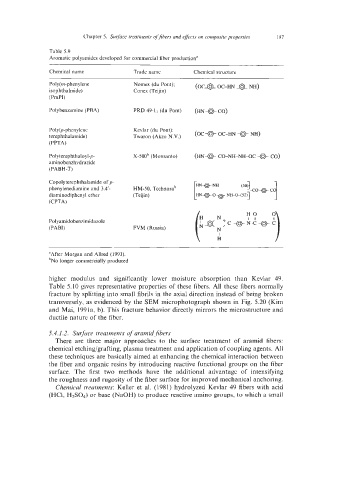
Table 5.9
Aromatic polyamides developed for commercial fiber production”
Chemical name Trade name Chemical structure
Pol y(m-phen ylene Nomex (du Pont); (oca OC-HN a
NH)
isophthalmide) Conex (Teijin)
(PmPI)
Polybenzdmine (PBA) PRD 49-1, (du Pont) (me CO)
Poly@-phenylene Kevlar (du Pont); (OC * oC-HN e
terephthalamide) Twdron (Akzo N.V.) NH)
(PPTA)
CO-NH-NH-OC e
Polyterephthaloyl-p- X-500b (Monsanto) (HN* CO)
aminobenzh ydrazide
(PABH-T)
Copolyterephthalamide of p-
phenylenediamine and 3,4‘- HM-50, Technorab
diaminodiphenyl ether (Teijin)
(CPTA)
“After Morgan and Ailred (1993).
hNo longer commercially produced
higher modulus and significantly lower moisture absorption than Kevlar 49.
Table 5.10 gives representative properties of these fibers. All these fibers normally
fracture by splitting into small fibrils in the axial direction instead of being broken
transversely, as evidenced by the SEM microphotograph shown in Fig. 5.20 (Kim
and Mai, 1991a, b). This fracture behavior directly mirrors the microstructure and
ductile nature of the fiber.
5.4.1.2. Surface treatments of ararnidJihers
There are three major approaches to the surface treatment of aramid fibers:
chemical etching/grafting, plasma treatment and application of coupling agents. All
these techniques are basically aimed at enhancing the chemical interaction between
the fiber and organic resins by introducing reactive functional groups on the fiber
surface. The first two methods have the additional advantage of intensifying
the roughness and rugosity of the fiber surface for improved mechanical anchoring.
Chemical treatments: Keller et al. (1981) hydrolyzed Kevlar 49 fibers with acid
(HC1, H2S04) or base (NaOH) to produce reactive amino groups, to which a small