Page 230 - Engineered Interfaces in Fiber Reinforced Composites
P. 230
212 Engineered interfaces in ,fiber reinforced composites
It is also interesting to note that electroless plating of silver on carbon fiber
surface improves the wettability between molten aluminum and PAN based carbon
fibers using the liquid infiltration technique in vacuum (Warrier et al., 1993). This is
attributed to the formation of an eutectic phase between silver and aluminum, and
the silver coating of the fibers during processing.
Alloying addition techniques have also been used to improve the wetting of
carbon fibers by liquid copper (DeVincent, 1991; DeVincent and Michal, 1993a, b).
Among the alloying elements studied, Fe, La, Mn, Nb, Si, Ta and Ti do not wet the
H-490 carbon fibers for the alloying levels examined. On the contrary, additions of
Cr and V at 1 at. wt% are able to enhance the wetting behavior so that a contact
angle of 45" or less is produced. However, because of the difficulties associated with
dissolving V in molten Cu, a temperature of 1530°C is needed to achieve the same
degree of wetting. This makes the Cu-V alloy systems rather impractical for
fabrication. Energy dispersive spectroscopy (EDS), Auger electron spectroscopy
(AES) and X-ray diffraction analyzes show that a reaction layer, Cr3C2, is formed by
bulk diffusion of carbon and the alloying atoms through the reaction layer, which is
followed by surface diffusion of the alloying atoms along the reaction layer.
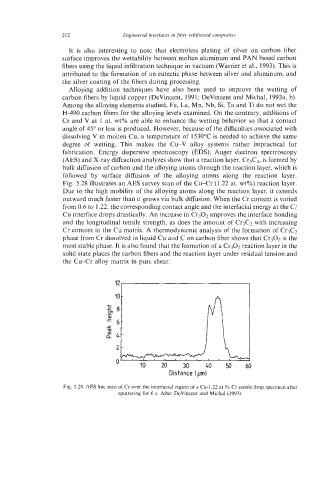
Fig. 5.28 illustrates an AES survey scan of the Cu-Cr (1.22 at. wt%) reaction layer.
Due to the high mobility of the alloying atoms along the reaction layer, it extends
outward much faster than it grows via bulk diffusion. When the Cr content is varied
from 0.6 to 1.22, the corresponding contact angle and the interfacial energy at the C/
Cu interface drops drastically. An increase in Cr302 improves the interface bonding
and the longitudinal tensile strength, as does the amount of Cr3C2 with increasing
Cr content in the Cu matrix. A thermodynamic analysis of the formation of Cr3C2
phase from Cr dissolved in liquid Cu and C on carbon fiber shows that Cr302 is the
most stable phase. It is also found that the formation of a Cr302 reaction layer in the
solid state places the carbon fibers and the reaction layer under residual tension and
the Cu-Cr alloy matrix in pure shear.
aJ.
6.
Y .
m
d 4-
2
n
v
10 20 30 40 50 60
Distance (pm)
Fig. 5.28. AES line scan of Cr over the interfacial region of a Cu-1.22 at.% Cr sessile drop specimen after
sputtering for 6 s. After DeVincent and Michal (1993).