Page 334 - Engineering Plastics Handbook
P. 334
292 Engineering Plastics
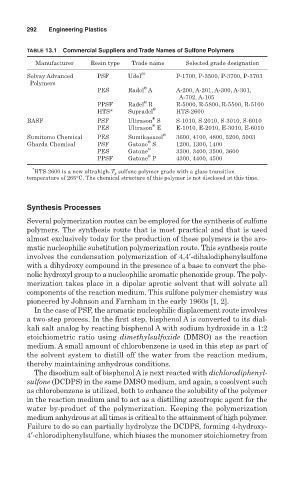
TABLE 13.1 Commercial Suppliers and Trade Names of Sulfone Polymers
Manufacturer Resin type Trade name Selected grade designation
Solvay Advanced PSF Udel (® P-1700, P-3500, P-3700, P-3703
Polymers
®
PES Radel A A-200, A-201, A-300, A-301,
A-702, A-105
®
PPSF Radel R R-5000, R-5800, R-5500, R-5100
®
HTS* Supradel HTS-2600
®
BASF PSF Ultrason S S-1010, S-2010, S-3010, S-6010
®
PES Ultrason E E-1010, E-2010, E-3010, E-6010
Sumitomo Chemical PES Sumikaexcel ® 3600, 4100, 4800, 5200, 5003
®
Gharda Chemical PSF Gatone S 1200, 1300, 1400
PES Gatone ® 3300, 3400, 3500, 3600
®
PPSF Gatone P 4300, 4400, 4500
*
HTS-2600 is a new ultrahigh-T g sulfone polymer grade with a glass transition
temperature of 265°C. The chemical structure of this polymer is not disclosed at this time.
Synthesis Processes
Several polymerization routes can be employed for the synthesis of sulfone
polymers. The synthesis route that is most practical and that is used
almost exclusively today for the production of these polymers is the aro-
matic nucleophilic substitution polymerization route. This synthesis route
involves the condensation polymerization of 4,4′-dihalodiphenylsulfone
with a dihydroxy compound in the presence of a base to convert the phe-
nolic hydroxyl group to a nucleophilic aromatic phenoxide group. The poly-
merization takes place in a dipolar aprotic solvent that will solvate all
components of the reaction medium. This sulfone polymer chemistry was
pioneered by Johnson and Farnham in the early 1960s [1, 2].
In the case of PSF, the aromatic nucleophilic displacement route involves
a two-step process. In the first step, bisphenol A is converted to its dial-
kali salt analog by reacting bisphenol A with sodium hydroxide in a 1:2
stoichiometric ratio using dimethylsulfoxide (DMSO) as the reaction
medium. A small amount of chlorobenzene is used in this step as part of
the solvent system to distill off the water from the reaction medium,
thereby maintaining anhydrous conditions.
The disodium salt of bisphenol A is next reacted with dichlorodiphenyl-
sulfone (DCDPS) in the same DMSO medium, and again, a cosolvent such
as chlorobenzene is utilized, both to enhance the solubility of the polymer
in the reaction medium and to act as a distilling azeotropic agent for the
water by-product of the polymerization. Keeping the polymerization
medium anhydrous at all times is critical to the attainment of high polymer.
Failure to do so can partially hydrolyze the DCDPS, forming 4-hydroxy-
4′-chlorodiphenylsulfone, which biases the monomer stoichiometry from