Page 332 - Engineering Plastics Handbook
P. 332
290 Engineering Plastics
It was produced by using the aromatic nucleophilic polycondensation reac-
tion of bisphenol A with 4,4′-dichlorodiphenylsulfone, a process still used
today by the commercial producers of this polymer. This polymer, known
as polysulfone (PSF), has a glass transition temperature of about 185°C.
It is the oldest and most widely utilized member of the family of sulfone
polymers. Polysulfone and all the commercially available sulfone poly-
mers are completely amorphous and transparent in their natural state.
They have high glass transition temperatures T ranging from 185 to
g
265°C. The high heat resistance derived from the high T ’s, along with the
g
high thermal oxidative resistance derived from the aromatic backbone
structure, allows use of these polymers in demanding high-performance
applications where high temperatures are encountered for long durations.
In addition to the high-temperature capability, the sulfone polymers boast
outstanding hydrolytic stability as another of their chief performance fea-
tures. In the early 1980s, the second of the commercially important sul-
fone polymers, polyethersulfone (PES), was introduced by Imperial
Chemical Industries in the United Kingdom. This polymer has the fol-
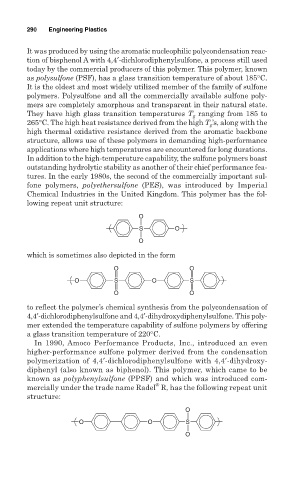
lowing repeat unit structure:
O
S O
O
which is sometimes also depicted in the form
O O
O S O S
O O
to reflect the polymer’s chemical synthesis from the polycondensation of
4,4′-dichlorodiphenylsulfone and 4,4′-dihydroxydiphenylsulfone. This poly-
mer extended the temperature capability of sulfone polymers by offering
a glass transition temperature of 220°C.
In 1990, Amoco Performance Products, Inc., introduced an even
higher-performance sulfone polymer derived from the condensation
polymerization of 4,4′-dichlorodiphenylsulfone with 4,4′-dihydroxy-
diphenyl (also known as biphenol). This polymer, which came to be
known as polyphenylsulfone (PPSF) and which was introduced com-
®
mercially under the trade name Radel R, has the following repeat unit
structure:
O
O O S
O