Page 335 - Engineering Plastics Handbook
P. 335
Polyarylethersulfones (PAES) 293
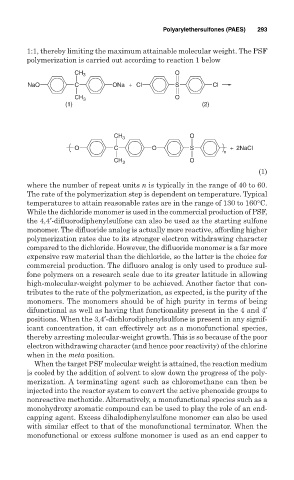
1:1, thereby limiting the maximum attainable molecular weight. The PSF
polymerization is carried out according to reaction 1 below
O
CH 3
NaO C ONa + Cl S Cl
CH 3 O
(1) (2)
CH 3 O
O C O S + 2NaCl
n
CH 3 O
(1)
where the number of repeat units n is typically in the range of 40 to 60.
The rate of the polymerization step is dependent on temperature. Typical
temperatures to attain reasonable rates are in the range of 130 to 160°C.
While the dichloride monomer is used in the commercial production of PSF,
the 4,4′-difluorodiphenylsulfone can also be used as the starting sulfone
monomer. The difluoride analog is actually more reactive, affording higher
polymerization rates due to its stronger electron withdrawing character
compared to the dichloride. However, the difluoride monomer is a far more
expensive raw material than the dichloride, so the latter is the choice for
commercial production. The difluoro analog is only used to produce sul-
fone polymers on a research scale due to its greater latitude in allowing
high-molecular-weight polymer to be achieved. Another factor that con-
tributes to the rate of the polymerization, as expected, is the purity of the
monomers. The monomers should be of high purity in terms of being
difunctional as well as having that functionality present in the 4 and 4′
positions. When the 3,4′-dichlorodiphenylsulfone is present in any signif-
icant concentration, it can effectively act as a monofunctional species,
thereby arresting molecular-weight growth. This is so because of the poor
electron withdrawing character (and hence poor reactivity) of the chlorine
when in the meta position.
When the target PSF molecular weight is attained, the reaction medium
is cooled by the addition of solvent to slow down the progress of the poly-
merization. A terminating agent such as chloromethane can then be
injected into the reactor system to convert the active phenoxide groups to
nonreactive methoxide. Alternatively, a monofunctional species such as a
monohydroxy aromatic compound can be used to play the role of an end-
capping agent. Excess dihalodiphenylsulfone monomer can also be used
with similar effect to that of the monofunctional terminator. When the
monofunctional or excess sulfone monomer is used as an end capper to