Page 339 - Engineering Plastics Handbook
P. 339
Polyarylethersulfones (PAES) 297
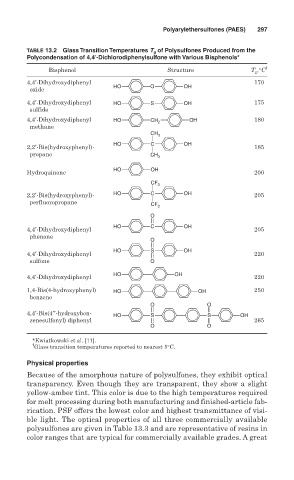
TABLE 13.2 Glass Transition Temperatures T g of Polysulfones Produced from the
Polycondensation of 4,4′-Dichlorodiphenylsulfone with Various Bisphenols*
Bisphenol Structure T g ,°C †
4,4′-Dihydroxydiphenyl 170
HO O OH
oxide
4,4′-Dihydroxydiphenyl HO S OH 175
sulfide
4,4′-Dihydroxydiphenyl HO CH 2 OH 180
methane
CH
3
HO C OH
2,2′-Bis(hydroxyphenyl)- 185
propane CH
3
HO OH
Hydroquinone 200
CF 3
2,2′-Bis(hydroxyphenyl)- HO C OH 205
perfluoropropane CF 3
O
HO C OH
4,4′-Dihydroxydiphenyl 205
phenone
O
HO S OH
4,4′-Dihydroxydiphenyl 220
sulfone O
HO OH
4,4′-Dihydroxydiphenyl 220
1,4-Bis(4-hydroxyphenyl) HO OH 250
benzene
O O
4,4′-Bis(4′′-hydroxyben-
HO S S OH
zenesulfonyl) diphenyl 265
O O
*Kwiatkowski et al. [11].
†
Glass transition temperatures reported to nearest 5°C.
Physical properties
Because of the amorphous nature of polysulfones, they exhibit optical
transparency. Even though they are transparent, they show a slight
yellow-amber tint. This color is due to the high temperatures required
for melt processing during both manufacturing and finished-article fab-
rication. PSF offers the lowest color and highest transmittance of visi-
ble light. The optical properties of all three commercially available
polysulfones are given in Table 13.3 and are representative of resins in
color ranges that are typical for commercially available grades. A great