Page 244 - Essentials of physical chemistry
P. 244
206 Essentials of Physical Chemistry
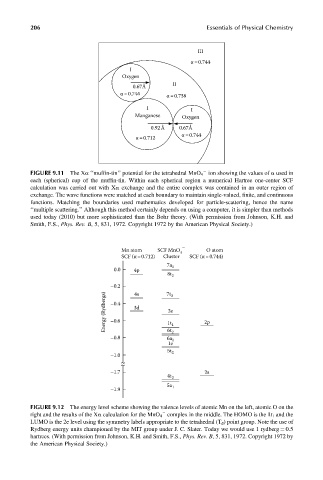
III
α =0.744
I
Oxygen
II
0.67Å
α= 0.744
α =0.738
I I
Manganese Oxygen
0.92Å 0.67Å
α =0.744
α= 0.712
FIGURE 9.11 The Xa ‘‘muffin-tin’’ potential for the tetrahedral MnO 4 ion showing the values of a used in
each (spherical) cup of the muffin-tin. Within each spherical region a numerical Hartree one-center SCF
calculation was carried out with Xa exchange and the entire complex was contained in an outer region of
exchange. The wave functions were matched at each boundary to maintain single-valued, finite, and continuous
functions. Matching the boundaries used mathematics developed for particle-scattering, hence the name
‘‘multiple scattering.’’ Although this method certainly depends on using a computer, it is simpler than methods
used today (2010) but more sophisticated than the Bohr theory. (With permission from Johnson, K.H. and
Smith, F.S., Phys. Rev. B, 5, 831, 1972. Copyright 1972 by the American Physical Society.)
–
Mn atom SCF MnO O atom
4
SCF (α=0.712) Cluster SCF (α=0.744)
0.0 7a 1
4p 8t 2
–0.2 4s 7t 2
Energy (Rydbergs) –0.4 3d 1t 2p
2e
–0.6
–0.8 6t 2 1
6a 1
1e
–1.0 5t 2
~
~
–1.7 4t 2 2s
–1.9 5a 1
FIGURE 9.12 The energy level scheme showing the valence levels of atomic Mn on the left, atomic O on the
right and the results of the Xa calculation for the MnO 4 complex in the middle. The HOMO is the 1t 1 and the
LUMO is the 2e level using the symmetry labels appropriate to the tetrahedral (T d ) point group. Note the use of
Rydberg energy units championed by the MIT group under J. C. Slater. Today we would use 1 rydberg ¼ 0.5
hartrees. (With permission from Johnson, K.H. and Smith, F.S., Phys. Rev. B, 5, 831, 1972. Copyright 1972 by
the American Physical Society.)