Page 358 - Excel for Scientists and Engineers: Numerical Methods
P. 358
335
CHAPTER 14 NONLINEAR REGRESSION USING THE SOLVER --
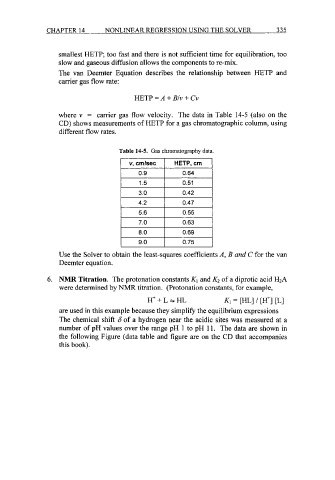
smallest HETP; too fast and there is not sufficient time for equilibration, too
slow and gaseous diffusion allows the components to re-mix.
The van Deemter Equation describes the relationship between HETP and
carrier gas flow rate:
HETP = A + 23/11 + Cv
where v = carrier gas flow velocity. The data in Table 14-5 (also on the
CD) shows measurements of HETP for a gas chromatographic column, using
different flow rates.
Table 14-5. Gas chromatography data.
v, cmlsec HETP, cm
0.9 0.64
I 1.5 I 0.51 I
3.0 0.42
4.2 0.47
I 5.6 I 0.55 I
7.0 0.63
8.0 0.69
9.0 0.75
6. NMR Titration. The protonation constants K1 and K2 of a diprotic acid H2A
were determined by NMR titration. (Protonation constants, for example,
H++L%HL K1= [HLI 1 WI [Ll
are used in this example because they simplify the equilibrium expressions
The chemical shift S of a hydrogen near the acidic sites was measured at a
number of pH values over the range pH 1 to pH 11. The data are shown in
the following Figure (data table and figure are on the CD that accompanies
this book).