Page 376 - Failure Analysis Case Studies II
P. 376
361
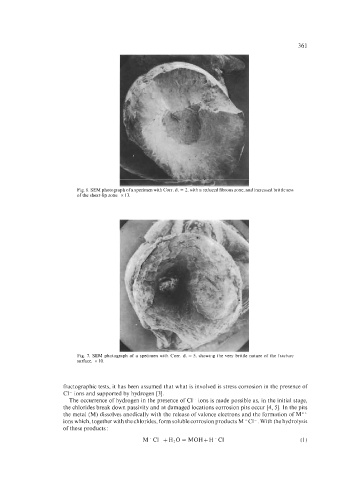
Fig. 6. SEM photograph of a specimen with Corr. d. = 2, with a reduced fibrous zone, and increased brittleness
of the shear-lip zone. x 13.
Fig. 7. SEM photograph of a specimen with Corr. d. = 5, showing the very brittle nature of the fracture
surface. x 10.
fractographic tests, it has been assumed that what is involved is stress corrosion in the presence of
C1- ions and supported by hydrogen [3].
The occurrence of hydrogen in the presence of C1- ions is made possible as, in the initial stage,
the chlorides break down passivity and at damaged locations corrosion pits occur [4, 51. In the pits
the metal (M) dissolves anodically with the release of valence electrons and the formation of M"+
ions which, together with the chlorides, form soluble corrosion products M +C1-. With the hydrolysis
of these products :
M+C1-+H20 = MOH+H+CI- (1)