Page 155 - Fluid Catalytic Cracking Handbook
P. 155
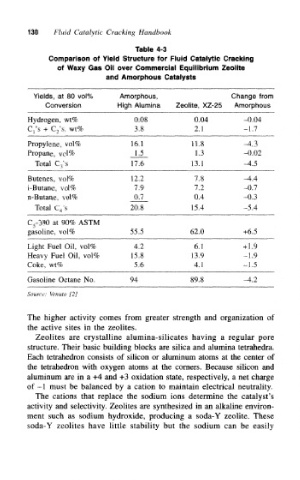
130 Fluid Catalytic Cracking Handbook
Table 4-3
Comparison of Yield Structure for Fluid Catalytic Cracking
of Waxy Gas Oil over Commercial Equilibrium Zeolite
and Amorphous Catalysts
Yields, at 80 vol% Amorphous, Change from
Conversion High Alumina Zeolite, XZ-25 Amorphous
Hydrogen, wt% 0.08 0.04 -0.04
C 1's + C 2's, wt% 3.8 2.1 -1.7
Propylene, vol% 16.1 11.8 -4.3
Propane, vol% 1.5 1.3 -0.02
Total C 3's 17.6 13.1 -4.5
Butenes, vol% 12.2 7.8 -4.4
i-Butane, vol% 7.9 7.2 -0.7
n-Butane, vol% 0,7 0.4 -0.3
Total C 4's 20.8 15.4 -5.4
C 5-390 at 90% ASTM
gasoline, vol% 55.5 62.0 +6.5
Light Fuel Oil, vol% 4.2 6.1 +1.9
Heavy Fuel Oil, vol% 15.8 13.9 -1.9
Coke, wt% 5.6 4.1 -1.5
Gasoline Octane No. 94 89.8 -4.2
Source: Venuto [2]
The higher activity comes from greater strength and organization of
the active sites in the zeolites.
Zeolites are crystalline alumina-silicates having a regular pore
structure. Their basic building blocks are silica and alumina tetrahedra.
Each tetrahedron consists of silicon or aluminum atoms at the center of
the tetrahedron with oxygen atoms at the corners. Because silicon and
aluminum are in a +4 and 4-3 oxidation state, respectively, a net charge
of -1 must be balanced by a cation to maintain electrical neutrality.
The cations that replace the sodium ions determine the catalyst's
activity and selectivity. Zeolites are synthesized in an alkaline environ-
ment such as sodium hydroxide, producing a soda-Y zeolite. These
soda-Y zeolites have little stability but the sodium can be easily