Page 353 - Fundamentals of Gas Shale Reservoirs
P. 353
MS OF GAS ADSORPTION ON MINERALS 333
(a) (b)
5
Na-Wyoming (T=353.5K) 100
CH 4 adsorption isotherm (mol/kg) 3 2 y=(a*b*x)/(1+b*x) CO 2 solubility (g CO 2 /100 g octane) 60
Langmuir fit
4
80
Weighting:
y
No weighting
40
Chi^2/Do = 0.00535
= 0.99859
R^2
b 0.11208 ± 0.00802
353 K
0
0 1 a 5.74426 ± 0.14703 20 323 K
0 5 10 15 20 25 30 2 4 6 8 10
P (MPa) P (MPa)
(c) (d)
4
800 Clay surface
CH 4 adsorption isotherm (mol/kg) 2 Partial density (kg/m 3 ) 600 CH 4
+
Na
3
400
1
Dry
Moist (7.15 wt% water)
T=353.5K 200
0 0
5 10 15 20 25 0.0 0.5 1.0 1.5 2.0 2.5
P (MPa) Z (nm)
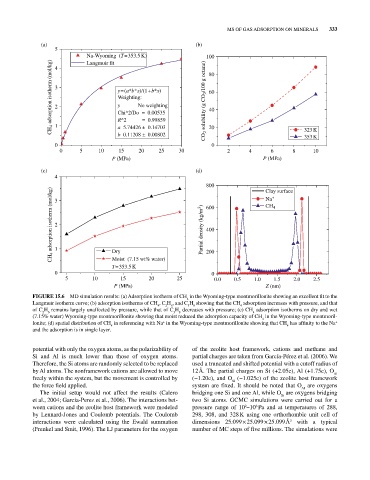
FIGURE 15.6 MD simulation results: (a) Adsorption isotherm of CH in the Wyoming‐type montmorillonite showing an excellent fit to the
4
Langmuir isotherm curve; (b) adsorption isotherms of CH , C H , and C H showing that the CH adsorption increases with pressure, and that
4
3
2
4
6
8
of C H remains largely unaffected by pressure, while that of C H decreases with pressure; (c) CH adsorption isotherms on dry and wet
3
8
6
2
4
(7.15% water) Wyoming‐type montmorillonite showing that moist reduced the adsorption capacity of CH in the Wyoming‐type montmoril
4
lonite; (d) spatial distribution of CH in referencing with Na in the Wyoming‐type montmorillonite showing that CH has affinity to the Na
+
+
4
4
and the adsorption is in single layer.
potential with only the oxygen atoms, as the polarizability of of the zeolite host framework, cations and methane and
Si and Al is much lower than those of oxygen atoms. partial charges are taken from García‐Pérez et al. (2006). We
Therefore, the Si atoms are randomly selected to be replaced used a truncated and shifted potential with a cutoff radius of
by Al atoms. The nonframework cations are allowed to move 12 Å. The partial charges on Si (+2.05e), Al (+1.75e), O
Al
freely within the system, but the movement is controlled by (−1.20e), and O (−1.025e) of the zeolite host framework
Si
the force field applied. system are fixed. It should be noted that O are oxygens
Al
The initial setup would not affect the results (Calero bridging one Si and one Al, while O are oxygens bridging
Si
et al., 2004; García‐Perez et al., 2006). The interactions bet two Si atoms. GCMC simulations were carried out for a
ween cations and the zeolite host framework were modeled pressure range of 10 –10 Pa and at temperatures of 288,
6
2
by Lennard‐Jones and Coulomb potentials. The Coulomb 298, 308, and 328 K using one orthorhombic unit cell of
interactions were calculated using the Ewald summation dimensions 25.099 × 25.099 × 25.099 Å with a typical
3
(Frenkel and Smit, 1996). The LJ parameters for the oxygen number of MC steps of five millions. The simulations were