Page 105 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 105
60 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
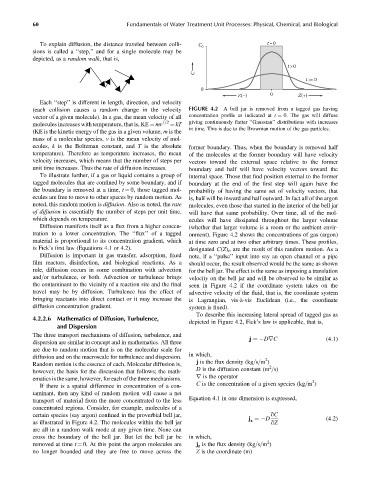
To explain diffusion, the distance traveled between colli- C 0 t=0
sions is called a ‘‘step,’’ and for a single molecule may be
depicted, as a random walk, that is,
t >0
C
t >> 0
0
Z(–) 0 Z(+)
Each ‘‘step’’ is different in length, direction, and velocity
(each collision causes a random change in the velocity FIGURE 4.2 A bell jar is removed from a tagged gas having
vector of a given molecule). In a gas, the mean velocity of all concentration profile as indicated at t ¼ 0. The gas will diffuse
1=2 giving continuously flatter ‘‘Gaussian’’ distributions with increases
molecules increases with temperature, that is, KE ¼ mv ¼ kT
in time. This is due to the Brownian motion of the gas particles.
(KE is the kinetic energy of the gas in a given volume, m is the
mass of a molecular species, v is the mean velocity of mol-
ecules, k is the Boltzman constant, and T is the absolute former boundary. Thus, when the boundary is removed half
temperature). Therefore as temperature increases, the mean of the molecules at the former boundary will have velocity
velocity increases, which means that the number of steps per vectors toward the external space relative to the former
unit time increases. Thus the rate of diffusion increases. boundary and half will have velocity vectors toward the
To illustrate further, if a gas or liquid contains a group of internal space. Those that find position external to the former
tagged molecules that are confined by some boundary, and if boundary at the end of the first step will again have the
the boundary is removed at a time, t ¼ 0, those tagged mol- probability of having the same set of velocity vectors, that
ecules are free to move to other spaces by random motion. As is, half will be inward and half outward. In fact all of the argon
noted, this random motion is diffusion. Also as noted, the rate molecules, even those that started in the interior of the bell jar
of diffusion is essentially the number of steps per unit time, will have that same probability. Over time, all of the mol-
which depends on temperature. ecules will have dissipated throughout the larger volume
Diffusion manifests itself as a flux from a higher concen- (whether that larger volume is a room or the ambient envir-
tration to a lower concentration. The ‘‘flux’’ of a tagged onment). Figure 4.2 shows the concentrations of gas (argon)
material is proportional to its concentration gradient, which at time zero and at two other arbitrary times. These profiles,
is Fick’s first law (Equations 4.1 or 4.2). designated C(Z) t , are the result of this random motion. As a
Diffusion is important in gas transfer, adsorption, fixed note, if a ‘‘pulse’’ input into say an open channel or a pipe
film reactors, disinfection, and biological reactions. As a should occur, the result observed would be the same as shown
rule, diffusion occurs in some combination with advection for the bell jar. The effect is the same as imposing a translation
and=or turbulence, or both. Advection or turbulence brings velocity on the bell jar and will be observed to be similar as
the contaminant to the vicinity of a reaction site and the final seen in Figure 4.2 if the coordinate system takes on the
travel may be by diffusion. Turbulence has the effect of advective velocity of the fluid, that is, the coordinate system
bringing reactants into direct contact or it may increase the is Lagrangian, vis-à-vis Euclidean (i.e., the coordinate
diffusion concentration gradient. system is fixed).
To describe this increasing lateral spread of tagged gas as
4.2.2.6 Mathematics of Diffusion, Turbulence,
depicted in Figure 4.2, Fick’s law is applicable, that is,
and Dispersion
The three transport mechanisms of diffusion, turbulence, and
j ¼ DrC (4:1)
dispersion are similar in concept and in mathematics. All three
are due to random motion that is on the molecular scale for
in which,
diffusion and on the macroscale for turbulence and dispersion.
2
j is the flux density (kg=s=m )
Random motion is the essence of each. Molecular diffusion is,
2
D is the diffusion constant (m =s)
however, the basis for the discussion that follows; the math-
ematics is the same, however, for each of the three mechanisms. r is the operator 3
If there is a spatial difference in concentration of a con- C is the concentration of a given species (kg=m )
taminant, then any kind of random motion will cause a net
transport of material from the more concentrated to the less Equation 4.1 in one dimension is expressed,
concentrated regions. Consider, for example, molecules of a
certain species (say argon) confined in the proverbial bell jar, qC
j ¼ D (4:2)
as illustrated in Figure 4.2. The molecules within the bell jar z qZ
are all in a random walk mode at any given time. None can
cross the boundary of the bell jar. But let the bell jar be in which,
2
removed at time t ¼ 0. At this point the argon molecules are j z is the flux density (kg=s=m )
no longer bounded and they are free to move across the Z is the coordinate (m)