Page 143 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 143
98 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
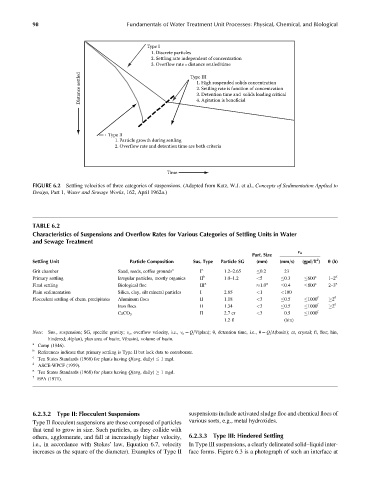
Type I
1. Discrete particles
2. Settling rate independent of concentration
3. Overflow rate=distance settled/time
Distance settled Type III
1. High suspended solids concentration
2. Settling rate is function of concentration
3. Detention time and solids loading critical
4. Agitation is beneficial
Type II
1. Particle growth during settling
2. Overflow rate and detention time are both criteria
Time
FIGURE 6.2 Settling velocities of three categories of suspensions. (Adapted from Katz, W.J. et al., Concepts of Sedimentation Applied to
Design, Part 1, Water and Sewage Works, 162, April 1962a.)
TABLE 6.2
Characteristics of Suspensions and Overflow Rates for Various Categories of Settling Units in Water
and Sewage Treatment
v o
Part. Size
2
Settling Unit Particle Composition Sus. Type Particle SG (mm) (mm=s) (gpd=ft ) u (h)
Grit chamber Sand, seeds, coffee grounds a I a 1.2–2.65 0.2 23
Primary settling Irregular particles, mostly organics II b 1.0–1.2 <5 0.3 600 c 1–2 d
a a c e
Final settling Biological floc III 1.0 0.4 800 2–3
Plain sedimentation Silica, clay, silt mineral particles I 2.65 <1 <100
f f
Flocculent settling of chem. precipitates Aluminum flocs II 1.18 <3 0.5 1000 2
Iron flocs II 1.34 <3 0.5 1000 f 2 f
II 2.7 cr <3 0.5 1000 f
CaCO 3
1.2 fl (hin)
Note: Sus., suspension; SG, specific gravity; v o , overflow velocity, i.e., v o ¼ Q=V(plan); u, detention time, i.e., u ¼ Q=A(basin); cr, crystal; fl, floc; hin,
hindered; A(plan), plan area of basin; V(basin), volume of basin.
a
Camp (1946).
b
References indicate that primary settling is Type II but lack data to corroborate.
c
Ten States Standards (1968) for plants having Q(avg. daily) 1 mgd.
d
ASCE-WPCF (1959).
e
Ten States Standards (1968) for plants having Q(avg. daily) 1 mgd.
f
EPA (1971).
6.2.3.2 Type II: Flocculent Suspensions suspensions include activated sludge floc and chemical flocs of
Type II flocculent suspensions are those composed of particles various sorts, e.g., metal hydroxides.
that tend to grow in size. Such particles, as they collide with
others, agglomerate, and fall at increasingly higher velocity, 6.2.3.3 Type III: Hindered Settling
i.e., in accordance with Stokes’ law, Equation 6.7, velocity In Type III suspensions, a clearly delineated solid–liquid inter-
increases as the square of the diameter). Examples of Type II face forms. Figure 6.3 is a photograph of such an interface at