Page 214 - Gas Purification 5E
P. 214
200 Gas PuniJication
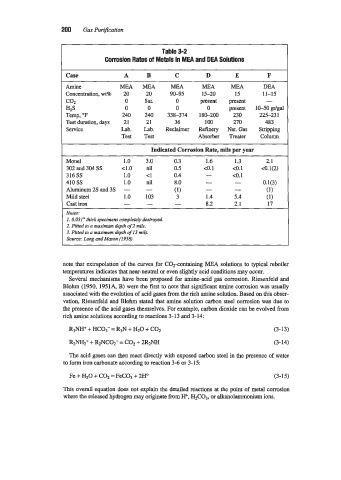
Table 3-2
Corrosion Rates of Metals in MEA and DEA Solutions
Case A B C D E P
~~~ ~
Amine MEA MEA MEA MEA MEA DEA
Concentration, wt% 20 20 90-95 15-20 15 11-15
COZ 0 Sat. 0 present present -
HZS 0 0 0 0 present 10-50 gr/gal
Temp, "F 240 240 338-374 180-200 230 225-23 1
Test duration, days 21 21 36 100 270 483
Service Lab. Lab. Reclaimer Refinery Nat. Gas Stripping
Test Test Absorber Treater Column
Indicated Corrosion Rate, mils per year
Monel 1.0 3.0 0.3 1.6 1.3 2.1
302 and 304 SS 4.0 nil 0.5 <o. 1 co. 1 <0.1(2)
316 SS 1.0 <1 0.4 - co. 1
410 SS 1.0 nil 8.0 - - 0.1(3)
Aluminum 2s and 3s - - (1) - - (1)
Mild steel 1.0 103 3 1.4 5.4 (1)
Cast iron - - - 8.2 2.1 17
Notes:
1. 0.031" thick specimens completely destroyed.
2. Pitted to a maximum depth of 2 mils.
3. Pitted to a maximum depth of 13 mils.
Source: Lang and Mason (1958)
note that extrapolation of the curves for COz-containing MEA solutions to typical reboiler
temperatures indicates that near-neutral or even slightly acid conditions may occur.
Several mechanisms have been proposed for amine-acid gas corrosion. Riesenfeld and
Blohm (1950, 1951A, B) were the first to note that significant amine corrosion was usually
associated with the evolution of acid gases from the rich amine solution. Based on this obser-
vation, Riesenfeld and Blohm stated that amine solution carbon steel corrosion was due to
the presence of the acid gases themselves. For example, carbon dioxide can be evolved from
rich amine solutions according to reactions 3-13 and 3-14:
R3NH+ + HC03- = R3N + HzO + COz (3-13)
RzNHZ+ + RzNCOz- = COZ + 2RzNH (3-14)
The acid gases can then react directly with exposed carbon steel in the presence of water
to form iron carbonate according to reaction 3-6 or 3-15:
Fe + H20 + COz = FeC03 + 2H" (3-15)
This overall equation does not explain the detailed reactions at the point of metal corrosion
where the released hydrogen may originate from H+, H2C03, or alkanolammoniurn ions.