Page 215 - Gas Purification 5E
P. 215
Mechanical Design and Operation of Alkanolamine Plants 201
I
n
TEMPERATURE OF
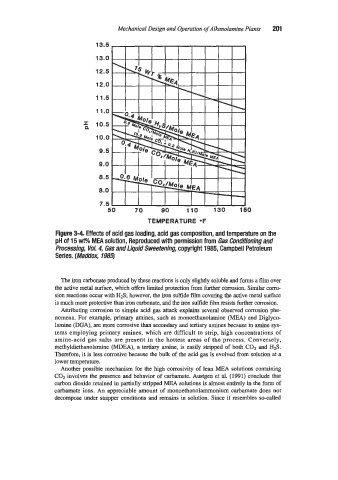
Figure 3-4. Effects of acid gas loading, acid gas composition, and temperature on the
pH of 15 WO MEA solution, Reproduced with permission from Gas Conditioning and
Processing, VoL 4, Gas and Liquid Sweetening, copyright 1985, Campbell Petroleum
Series. (Maddox, 1985)
The iron carbonate produced by these reactions is only slightly soluble and forms a film over
the active metal surface, which offers limited protection from further corrosion. Similar corro-
sion reactions occur with H2S; however, the iron sulfide film covering the active metal surface
is much more protective than iron carbonate, and the iron sulfide film resists further corrosion.
Attributing corrosion to simple acid gas attack explains several observed corrosion phe-
nomena. For example, primary amines, such as monoethanolamine (MEA) and Diglyco-
lamine (DGA), are more corrosive than secondary and tertiary amines because in amine sys-
tems employing primary amines, which are difficult to strip, high concentrations of
amine-acid gas salts are present in the hottest areas of the process. Conversely,
methyldiethanolamine (MDEA), a tertiary amine, is easily stripped of both C02 and H2S.
Therefore, it is less corrosive because the bulk of the acid gas is evolved from solution at a
lower temperature.
Another possible mechanism for the high corrosivity of lean MEA solutions containing
COz involves the presence and behavior of carbamate. Austgen et al. (1991) conclude that
carbon dioxide retained in partially stripped MEA solutions is almost entirely in the form of
carbamate ions. An appreciable amount of monoethanolammonium carbamate does not
decompose under stripper conditions and remains in solution. Since it resembles so-called