Page 273 - Gas Purification 5E
P. 273
Mechanical Design and Operation of Alkanolamine Plants 257
In the first reaction, the carbonate anion liberates one mole of amine per mole of carbonate
anion. In the second, another mole of amine is liberated by the one mole of bicarbonate
anion produced by the frst reaction. One mole of sodium carbonate, therefore, neutralizes
two moles of bound amine.
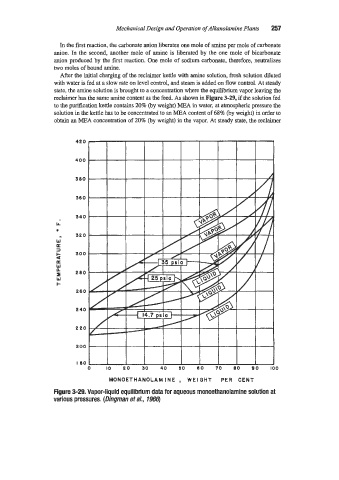
After the iuitial charging of the reclaimer kettle with amine solution, fresh solution diluted
with water is fed at a slow rate on level control, and steam is added on flow control. At steady
state, the amine solution is brought to a concentration where the equilibrium vapor leaving the
reclaimer has the same amine content as the feed. As shown in Figure 3-29, if the solution fed
to the purification kettle contains 20% (by weight) MEA in water, at atmospheric pressure the
solution in the kettle has to be collcentrated to an MEA content of 68% (by weight) in order to
obtain an MEA concentration of 20% (by weight) in the vapor. At steady state, the reclaimer
O 10 20 30 40 50 60 70 80 SO 100
MONOETHANOLAM INE , WEIGHT PER CENT
Rgure 3-29. Vapor-liquid equilibrium data for aqueous monoethanolamine solution at
various pressures. (Dingman et a/-, 7966)