Page 301 - Gas Purification 5E
P. 301
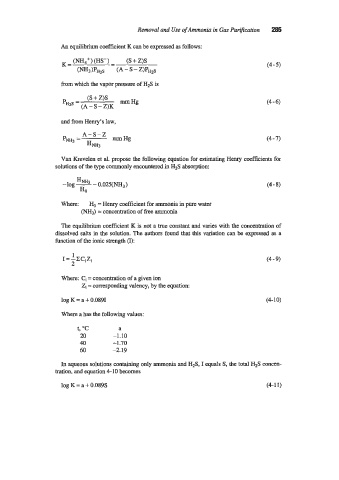
Removal and Use of Ammonia in Gas Purification 285
An equilibrium coefficient K can be expressed as follows:
from which the vapor pressure of H2S is
(S + Z)S
-
pHzS = Hg (4 6)
(A - S- Z)K
and from Henry's law,
Van Krevelen et al. propose the following equation for estimating Henry coefficients for
solutions of the type commonly encountered in H2S absorption:
HNH3
-log - - O.O25(NH,) (4-8)
HO
Where: H,, = Henry coefficient for ammonia in pure water
(NH3) = concentration of free ammonia
The equilibrium coefficient K is not a true constant and varies with the concentration of
dissolved salts in the solution. The authors found that this variation can be expressed as a
function of the ionic strength (I):
1
I = --zcizj (4 9)
-
2
Where: Ci = concentration of a given ion
Z, = corresponding valency, by the equation:
log K = a + 0.0891 (4- 10)
Where a has the following values:
t, "C a
20 -1.10
40 -1.70
60 -2.19
In aqueous solutions containing only ammonia and H2S, I equals S, the total H2S concen-
tration, and equation 4-10 becomes
log K = a + 0.089s (4-1 1)