Page 300 - Gas Purification 5E
P. 300
284 Gas Purification
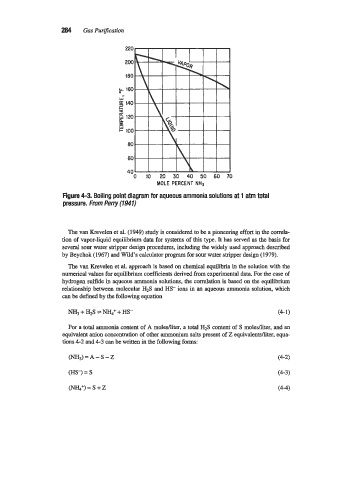
Figure 4-3. Boiling point diagram for aqueous ammonia solutions at 1 atm total
pressure. From Perry (1941)
The van Krevelen et al. (1949) study is considered to be a pioneering effort in the correla-
tion of vapor-liquid equilibrium data for systems of this type. It has served as the basis for
several sour water stripper design procedures, including the widely used approach described
by Beychok (1967) and Wild's calculator program for sour water stripper design (1979).
The van Krevelen et al. approach is based on chemical equilibria in the solution with the
numerical values for equilibrium coefficients derived from experimental data. For the case of
hydrogen sulfide in qumus ammonia solutions, the correlation is based on the equilibrium
relationship between molecular H2S and HS- ions in an aqueous ammonia solution, which
can be defined by the following equation
NH3 + H2S N&+ + HS- (4-1)
For a total ammonia content of A molesfliter, a total H2S content of S molesfliter, and an
equivalent anion concentration of other ammonium salts present of Z equivalentsfliter, equa-
tions 4-2 and 4-3 can be written in the following forms:
=
(HS-) S (4-3)
(Nr&+) = s + z (4-4)