Page 298 - Gas Purification 5E
P. 298
282 Gas Purification
before the advent of synthetic ammonia, when coal gas constituted the largest source of fmed
nitrogen.
Carbon dioxide is also a major impurity in coal gas. Although it is not generally necessary
to remove COP from gases used as fuels, partial removal is sometimes desirable to improve
the heating value. Complete C02 elimination is required for gases undergoing processing at
very low temperatures, for example, in cokeoven gas purification to provide hydrogen for
ammonia synthesis. The removal of other impurities such as HCN, COS, organic acids, and
pyridine may also be required when the concentration is high enough to cause pollution or
operating problems in downstream systems.
As indicated by the data of Table 4-4, gases produced by oxygen-blown gasifiers general-
ly contain appreciably less ammonia than coke oven gas. As a result, the use of aqueous
ammonia has not been developed as a gas purification technique for such gases. Gases pro-
duced by air-blown gasifiers and by the thermal treatment of petroleum streams (including
shale oil and tar sands liquids) are also relatively low in ammonia and are not considered to
be appropriate candidates for ammonia-based scrubbing processes.
Sources of Sour Water
In refinery catalytic cracking operations, oxygen-blown coal gasifiers and similar high
temperature processes that produce a gas stream containing both ammonia and hydrogen sul-
fide, the gas leaving the high temperature step is typically quenched with water or cooled by
indirect heat exchange and then scrubbed with water. The resulting “sour water” contains
essentially all of the ammonia, but normally contains only a small portion of the H2S and
C02 contained in the gas stream. It also contains water soluble impurities such as organic
acids and phenols. Considerable development work has gone into the processing of sour
water. Because its processing constitutes an essential step in the overall gas purification
scheme, and ammonia is always one component, key processes for sour water treatment are
described in the Ammonia Removal section of this chapter.
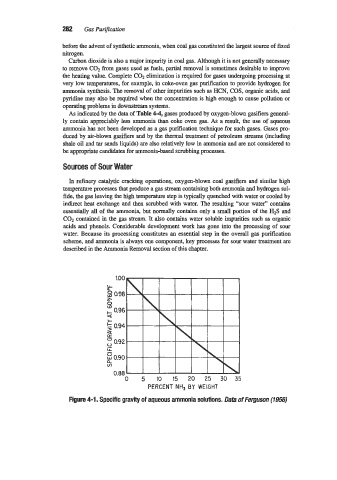
PERCENT NH, BY WEIGHT
Figure 4-1. Specific gravity of aqueous ammonia solutions. Data of Fernuson (795s)