Page 297 - Gas Purification 5E
P. 297
Removal and Use of Ammonia in Gas Purijication 281
Coal Gas Impurities
Ammonia, hydrogen sulfide, and carbon dioxide are major impurities in coke oven gas
(COG). In addition to these three components, COG often contains carbon disulfide, carbonyl
sulfide, hydrogen cyanide, organic acids, pyridine, phenol, and other impurities, which can
cause problems with conventional amine plants. The presence of large quantities of ammonia in
these gases naturally led to consideration of its use for removal of H2S and COz, and several
ammonia-based coke oven gas purification processes have been developed and commercialid.
Processes that use aqueous ammonia to remove HzS and COz are still offered for coke-
oven gas purification, and many such plants are in operation in the U.S. and Europe; howev-
er, it appears that the trend for new operations is toward the use of other absorbents. Other
absorption processes that may be applicable for COG purification include the Takahax,
Stretford, Vacuum Carbonate, Potassium Carbonate, and Sulfiban (MEA) processes. These
processes can be designed to avoid serious adverse effects of trace impurities in the gas, and
generally provide somewhat higher H2S removal efficiency than ammonia scrubbing. The
processes are described in detail in other chapters (see index).
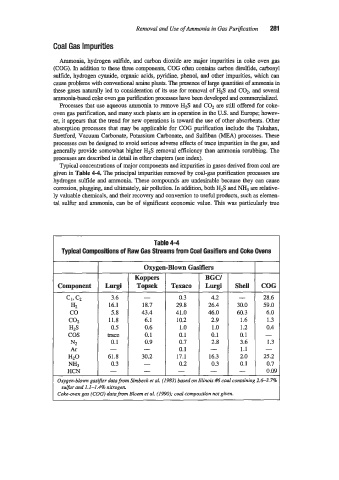
Typical concentrations of major components and impurities in gases derived from coal are
given in Table 4-4. The principal impurities removed by coal-gas purification processes are
hydrogen sulfide and ammonia. These compounds are undesirable because they can cause
corrosion, plugging, and ultimately, air pollution. In addition, both H2S and NH3 are relative-
ly valuable chemicals, and their recovery and conversion to useful products, such as elemen-
tal sulfur and ammonia, can be of significant economic value. This was particularly true
Table 4-4
Typical Compositions of Raw Gas Streams from Coal Gasifiers and Coke Ovens
Koppers BGCI
Component Lurgi Topsek Texaco Lurgi Shell COG
CI, cz 3.6 - 0.3 4.2 - 28.6
HZ 16.1 18.7 29.8 26.4 30.0 59.0
co 5.8 43.4 41.0 46.0 60.3 6.0
COZ 11.8 6.1 10.2 2.9 1.6 1.3
HZS 0.5 0.6 1 .o 1 .o 1.2 0.4
cos trace 0.1 0.1 0.1 0.1 -
N2 0.1 0.9 0.7 2.8 3.6 1.3
Ar - - 0.1 - 1.1 -
HZO 61.8 30.2 17.1 16.3 2.0 25.2
NH3 0.3 - 0.2 0.3 0.1 0.7
HCN - - - - - 0.09
Oxygen-blown gasij?er datafrom Simbeck et al. (1983) based on Illinois #6 coal containing 2.6-3.7%
sulfur and 1.1-1.496 nitrogen
Coke-oven gas (COG) data from Bloem et al. (1990); coal composition not given.