Page 126 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 126
110 Gas Wettability of Reservoir Rock Surfaces with Porous Media
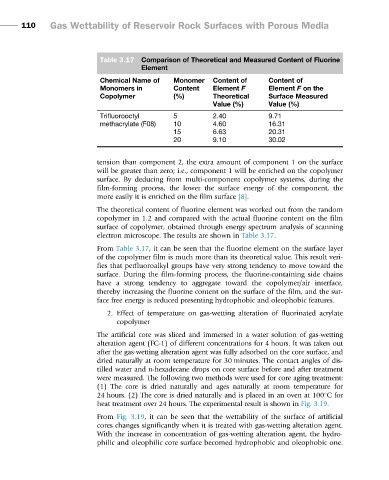
Table 3.17 Comparison of Theoretical and Measured Content of Fluorine
Element
Chemical Name of Monomer Content of Content of
Monomers in Content Element F Element F on the
Copolymer (%) Theoretical Surface Measured
Value (%) Value (%)
Trifluorooctyl 5 2.40 9.71
methacrylate (F08) 10 4.60 16.31
15 6.63 20.31
20 9.10 30.02
tension than component 2, the extra amount of component 1 on the surface
will be greater than zero; i.e., component 1 will be enriched on the copolymer
surface. By deducing from multi-component copolymer systems, during the
film-forming process, the lower the surface energy of the component, the
more easily it is enriched on the film surface [8].
The theoretical content of fluorine element was worked out from the random
copolymer in 1.2 and compared with the actual fluorine content on the film
surface of copolymer, obtained through energy spectrum analysis of scanning
electron microscope. The results are shown in Table 3.17.
From Table 3.17, it can be seen that the fluorine element on the surface layer
of the copolymer film is much more than its theoretical value. This result veri-
fies that perfluoroalkyl groups have very strong tendency to move toward the
surface. During the film-forming process, the fluorine-containing side chains
have a strong tendency to aggregate toward the copolymer/air interface,
thereby increasing the fluorine content on the surface of the film, and the sur-
face free energy is reduced presenting hydrophobic and oleophobic features.
2. Effect of temperature on gas-wetting alteration of fluorinated acrylate
copolymer
The artificial core was sliced and immersed in a water solution of gas-wetting
alteration agent (FC-1) of different concentrations for 4 hours. It was taken out
after the gas-wetting alteration agent was fully adsorbed on the core surface, and
dried naturally at room temperature for 30 minutes. The contact angles of dis-
tilled water and n-hexadecane drops on core surface before and after treatment
were measured. The following two methods were used for core aging treatment:
(1) The core is dried naturally and ages naturally at room temperature for
24 hours. (2) The core is dried naturally and is placed in an oven at 100 Cfor
heat treatment over 24 hours. The experimental result is shown in Fig. 3.19.
From Fig. 3.19, it can be seen that the wettability of the surface of artificial
cores changes significantly when it is treated with gas-wetting alteration agent.
With the increase in concentration of gas-wetting alteration agent, the hydro-
philic and oleophilic core surface becomed hydrophobic and oleophobic one.