Page 120 - Gas Adsorption Equilibria
P. 120
106 Chapter 2
5.3 Example
Integral heats of adsorption and Gibbs excess masses of n-butane
adsorbed on activated carbon (AC BAX 1100) have been measured
simultaneously in a SGC at various temperatures for pressures up to 0.2 MPa.
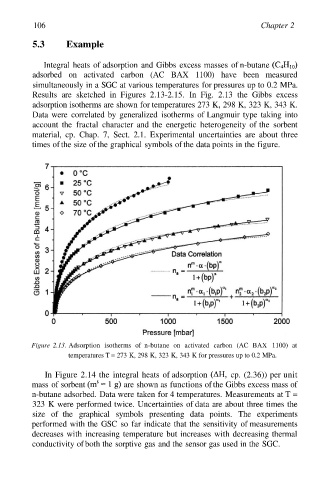
Results are sketched in Figures 2.13-2.15. In Fig. 2.13 the Gibbs excess
adsorption isotherms are shown for temperatures 273 K, 298 K, 323 K, 343 K.
Data were correlated by generalized isotherms of Langmuir type taking into
account the fractal character and the energetic heterogeneity of the sorbent
material, cp. Chap. 7, Sect. 2.1. Experimental uncertainties are about three
times of the size of the graphical symbols of the data points in the figure.
Figure 2.13. Adsorption isotherms of n-butane on activated carbon (AC BAX 1100) at
temperatures T = 273 K, 298 K, 323 K, 343 K for pressures up to 0.2 MPa.
In Figure 2.14 the integral heats of adsorption cp. (2.36)) per unit
mass of sorbent are shown as functions of the Gibbs excess mass of
n-butane adsorbed. Data were taken for 4 temperatures. Measurements at T =
323 K were performed twice. Uncertainties of data are about three times the
size of the graphical symbols presenting data points. The experiments
performed with the GSC so far indicate that the sensitivity of measurements
decreases with increasing temperature but increases with decreasing thermal
conductivity of both the sorptive gas and the sensor gas used in the SGC.