Page 121 - Gas Adsorption Equilibria
P. 121
2. Volumetry / Manometry 107
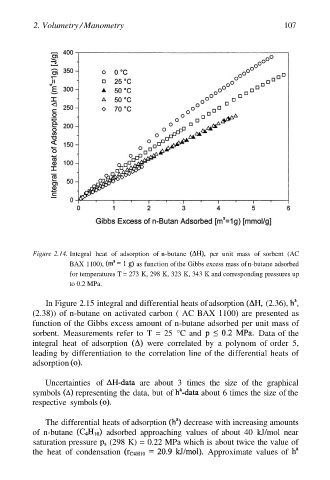
Figure 2.14. Integral heat of adsorption of n-butane per unit mass of sorbent (AC
BAX 1100), as function of the Gibbs excess mass of n-butane adsorbed
for temperatures T = 273 K, 298 K, 323 K, 343 K and corresponding pressures up
to 0.2 MPa.
In Figure 2.15 integral and differential heats of adsorption (2.36),
(2.38)) of n-butane on activated carbon ( AC BAX 1100) are presented as
function of the Gibbs excess amount of n-butane adsorbed per unit mass of
sorbent. Measurements refer to T = 25 °C and Data of the
integral heat of adsorption were correlated by a polynom of order 5,
leading by differentiation to the correlation line of the differential heats of
adsorption
Uncertainties of are about 3 times the size of the graphical
symbols representing the data, but of about 6 times the size of the
respective symbols
The differential heats of adsorption decrease with increasing amounts
of n-butane adsorbed approaching values of about 40 kJ/mol near
saturation pressure (298 K) = 0.22 MPa which is about twice the value of
the heat of condensation Approximate values of